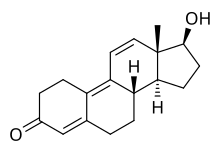
Trenbolone
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Archaeological site in Fars Province, Iran 29°57′59″N 52°53′13″E / 29.966486°N 52.887043°E / 29.966486; 52.887043 Shapur's Parade at Naqsh-e Rajab Naqsh-e Rajab (Persian: نقش رجب, Persian pronunciation: [næɣʃeɾæˈdʒæb]) is an archaeological site just west of Istakhr and about 5 km north of Persepolis in Fars province, Iran. Together with Naqsh-e Rostam, which lies 2.5 kilometres (1.6 mi) away, the site is part of the Marvdasht cu…

Algerian football club Football clubNRB Teleghma/BFull nameNadi Riadi Baladiate TeleghmaNickname(s)Zarga, Ouled SmailFounded1992; 32 years ago (1992)(as Nadi Riadi Baladiate Teleghma)GroundBachir Khabaza StadiumCapacity5000PresidentToufik BoudiafHead CoachRabah Zemamta[1]LeagueLigue 22022–23Ligue 2, Group Centre-east, 3rd Home colours Away colours Current season Nadi Riadi Baladiate Teleghma (Arabic: النادي الرياضي لبلدية التلاغمة), known…

У этого термина существуют и другие значения, см. Фугас. Под № 25 — Фугас, в правом нижнем углу иллюстрации Фугас камнемётный или камнемёт Фугас камнемётный или камнемёт, вид сверху Артиллерийские снаряды XIX века (в порядке расположения на рисунке): Верхний ряд: 1-3 …

Inlet in southeast Norway For other uses, see Oslofjord (disambiguation).OslofjordOslofjordenMap of the inletLocationSoutheast NorwayCoordinates59°48′08″N 10°33′09″E / 59.80229°N 10.55237°E / 59.80229; 10.55237TypeFjordPrimary outflowsSkaggerakBasin countriesNorwayMax. length120 kilometres (75 mi)SettlementsOslo The Oslofjord (Norwegian: Oslofjorden, Urban East Norwegian: [ˈʊ̂ʂlʊˌfjuːɳ]; English: Oslo Fjord[1][2][3…

Voce principale: Sportgemeinschaft 09 Wattenscheid. Sportgemeinschaft 09 WattenscheidStagione 1974-1975Sport calcio Squadra Wattenscheid 09 Allenatore Karlheinz Feldkamp 2. Bundesliga7º posto Coppa di GermaniaPrimo turno Maggiori presenzeCampionato: Jendrossek, Klee, Klimke (38)Totale: Klee, Klimke (39) Miglior marcatoreCampionato: Jendrossek (16)Totale: Jendrossek (16) StadioLohrheidestadion Maggior numero di spettatori12 000 vs. Borussia Dortmund Minor numero di spettatori1 500…

「俄亥俄」重定向至此。关于其他用法,请见「俄亥俄 (消歧义)」。 俄亥俄州 美國联邦州State of Ohio 州旗州徽綽號:七葉果之州地图中高亮部分为俄亥俄州坐标:38°27'N-41°58'N, 80°32'W-84°49'W国家 美國加入聯邦1803年3月1日,在1953年8月7日追溯頒定(第17个加入联邦)首府哥倫布(及最大城市)政府 • 州长(英语:List of Governors of {{{Name}}}]]) • …

Public high school in Davao City, Philippines Philippine Science High School Southern Mindanao CampusAddressSto. Niño, Tugbok DistrictDavao CityPhilippinesCoordinates7°05′02″N 125°30′29″E / 7.0837984°N 125.5081127°E / 7.0837984; 125.5081127InformationTypespecialized public high schoolEstablishedJuly 8, 1988 (1988-07-08)Campus DirectorDr. Jonald P. FeneciosGrades7 to 12Enrollment358 (SY 2009-2010); 361 (SY 2007-2008)NicknamePisayAffiliationDepar…

President of Peru from 2021 to 2022 For other people named Pedro Castillo, see Pedro Castillo (disambiguation). In this Spanish name, the first or paternal surname is Castillo and the second or maternal family name is Terrones. Pedro CastilloOSP OCACastillo in 202263rd President of PeruIn office28 July 2021 – 7 December 2022Prime MinisterGuido BellidoMirtha VásquezHéctor ValerAníbal TorresBetssy ChávezVice PresidentFirst Vice PresidentDina BoluarteSecond Vice PresidentVaca…
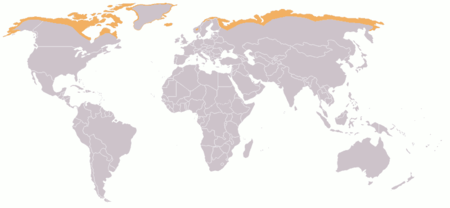
Biome where plant growth is hindered by frigid temperatures For other uses, see Tundra (disambiguation). TundraTundra in GreenlandMap showing Arctic tundraGeographyArea11,563,300[1] km2 (4,464,600 sq mi)Climate typeET In physical geography, tundra (/ˈtʌndrə, ˈtʊn-/) is a type of biome where tree growth is hindered by frigid temperatures and short growing seasons. The term is a Russian word adapted from Sámi languages.[2] There are three regions and associated…

Hong Kong TV series or program The Legend of the Condor HeroesDVD cover artChinese nameTraditional Chinese射鵰英雄傳Simplified Chinese射雕英雄传TranscriptionsStandard MandarinHanyu PinyinShè Diāo Yīng Xióng ZhuànYue: CantoneseJyutpingSe6 Diu1 Jing1 Hung4 Zyun6 GenreWuxiaBased onThe Legend of the Condor Heroesby Louis ChaScreenplay byWong Kwok-faiDirected byLeung Tak-wahYuen Ying-mingLam Kin-lungYun Wai-yeeKong Kam-hungLau Kwok-hoLam Dik-on (action director)StarringJulian Cheu…

Cet article est une ébauche concernant la Bretagne et l’histoire. Vous pouvez partager vos connaissances en l’améliorant (comment ?) selon les recommandations des projets correspondants. Chronologies Données clés 1886 1887 1888 1889 1890 1891 1892Décennies :1850 1860 1870 1880 1890 1900 1910Siècles :XVIIe XVIIIe XIXe XXe XXIeMillénaires :-Ier Ier IIe IIIe Chronologies géographiques Afrique Afrique du Sud, Algérie, A…

Mobile payment service developed by Block, Inc. Cash AppDeveloper(s)Block, Inc.Initial releaseOctober 15, 2013 (2013-10-15)Operating systemAndroid, iOSAvailable inEnglish, FrenchTypeMobile paymentWebsitecash.app Cash App (formerly Square Cash) is a mobile payment service available in the United States and the United Kingdom that allows users to transfer money to one another using a mobile phone app.[1] As of 2024, the service reports 57 million monthly transacting users an…

Эта статья — о деревне. Об улице в Москве см. Большая Семёновская улица. ДеревняБольшая Семёновская 59°59′56″ с. ш. 42°45′20″ в. д.HGЯO Страна Россия Субъект Федерации Вологодская область Муниципальный район Тотемский Сельское поселение Пятовское Истор…

Aleksandar ŠapićАлександар ШапићŠapić pada 2016 Walikota Beograd ke-75PetahanaMulai menjabat 20 Juni 2022PendahuluZoran RadojičićPenggantiPetahanaPresiden Kota Beograd BaruMasa jabatan27 Juni 2012 – 27 Juni 2022PendahuluNenad MilenkovićPenggantiBojan Bovan Informasi pribadiLahir1 Juni 1978 (umur 45)Beograd, SR Serbia, SFR YugoslaviaPartai politikDS (2006–2014)SPAS (2018–2021)SNS (2021–sekarang)Suami/istriMilica ŠapićAnak2Alma materUniversitas Meg…

British politician The Right HonourableThe Lord SoleyPCOfficial portrait, 2019Chair of the Parliamentary Labour PartyIn office3 May 1997 – 11 July 2001LeaderTony BlairPreceded byDoug HoyleSucceeded byJean CorstonMember of the House of LordsLord TemporalIn office29 June 2005 – 19 January 2023Life PeerageMember of Parliament for Ealing, Acton and Shepherd's BushHammersmith (1983–1997)Hammersmith North (1979–1983)In office3 May 1979 – 11 April 2005Preceded byFra…

Зміст 1 Список лауреатів 1.1 1900-ті 1.2 1910-ті 1.3 1920-ті 1.4 1930-ті 1.5 1940-ві 1.6 1950-ті 1.7 1960-ті 1.8 1970-ті 1.9 1980-ті 1.10 1990-ті 1.11 2000-ні 1.12 2010-ті 1.13 2020-ті 2 Див. також 3 Примітки 4 Література Нобелівська премія з фізики (швед. Nobelpriset i fysik) — вища нагорода за наукові досягнення в області фізики, щорічно…

American politician (1838–1911) This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Ezra C. Carleton – news · newspapers · books · scholar · JSTOR (May 2024) (Learn how and when to remove this message) Ezra C. CarletonC.M. Bell Studio Collection (Library of Congress), Circa 1882Member of the U.S. House of…

Stoples berisi madu dengan sendok madu dan biskuit Madu adalah subtansi makanan manis dan kental yang dibuat oleh lebah madu dan beberapa serangga lain.[1] Lebah menghasilkan madu dari sekresi gula tumbuhan (nektar bunga) atau dari sekresi serangga lain (seperti honeydew atau madu serangga). Madu terbentuk melalui regurgitasi, aktivitas enzimatik, dan penguapan air. Lebah menyimpan madu dalam struktur lilin yang disebut sarang lebah.[1][2] Variasi madu yang dihasilkan ole…

This article needs to be updated. Please help update this article to reflect recent events or newly available information. (August 2021) Prevalence of HIV/AIDS in Africa, total (% of population ages 15–49), in 2021 (World Bank) HIV/AIDS originated in the early 20th century and has become a major public health concern and cause of death in many countries. AIDS rates vary significantly between countries, with the majority of cases concentrated in Southern Africa. Although the continent is home t…

Museum Menara Gentala ArasyInformasiDidirikan pada2014Koordinat1°35′03″S 103°36′51″E / 1.58425°S 103.61409°E / -1.58425; 103.61409AlamatJembatan Pedestrian Sungai Batang Hari, Desa Arab Melayu, Kec. Pelayangan, Kota Jambi Pintu Masuk Museum Menara Gentala Arasy Jambi Museum Menara Gentala Arasy adalah museum yang menggambarkan perkembangan Islam di Provinsi Jambi. Museum Menara Gentala Arasy merupakan museum yang menyajikan perkembangan Islam di Jambi. Museum …