Harmine
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||
Read other articles:

Северный морской котик Самец Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Синапсиды…

国民阵线Barisan NasionalNational Frontباريسن ناسيونلபாரிசான் நேசனல்国民阵线标志简称国阵,BN主席阿末扎希总秘书赞比里署理主席莫哈末哈山总财政希山慕丁副主席魏家祥维纳斯瓦兰佐瑟古律创始人阿都拉萨成立1973年1月1日 (1973-01-01)[1]设立1974年7月1日 (1974-07-01)前身 联盟总部 马来西亚 吉隆坡 50480 秋傑区敦依斯迈路太子世贸中心(英语:…

South American Cricket ChampionshipFormatLimited-overs cricketFirst edition1995Latest editionMen: 2023Women: 2022Current championMen: Argentina (2023 – 12th title)Women: Brazil (2022 – 5th title)Most successfulMen: Argentina[a] (12 titles)Women: Argentina (7 titles) The South American Cricket Championship (Spanish: Campeonato Sudamericano de Críquet; Portuguese: Campeonato Sul-Americano de Críquete) is an international limited-overs cricket tournament featur…

Peta yang menunjukkan perubahan tembok kota di Beijing pada masa Dinasti Liao, Jin, Yuan, Ming, dan Qing Zhongdu (中都, secara harfiah berarti Ibu kota Pusat) adalah ibu kota Dinasti Jin yang dikuasai oleh bangsa Jurchen di Tiongkok pada abad pertengahan. Kota ini terletak di Distrik Xicheng di Beijing barat daya. Populasinya hampir mencapai satu juta jiwa pada akhir abad ke-12,[1] dan merupakan kota pra-modern terbesar dan terakhir yang dibangun di tempat tersebut.[2] Setelah …

Västergötlands FotbollförbundAbbreviationVästergötlands FFFormation17 March 1918PurposeDistrict Football AssociationHeadquartersTorggatan 8LocationBox 38354128 Skövde Västra Götaland County SwedenChairmanMagnus GunnarssonWebsitehttp://www.vastgotafotboll.org/ The Västergötlands Fotbollförbund (Västergötland Football Association) is one of the 24 district organisations of the Swedish Football Association. It administers lower tier football in the historical province of Västergötlan…

Artikel ini membutuhkan rujukan tambahan agar kualitasnya dapat dipastikan. Mohon bantu kami mengembangkan artikel ini dengan cara menambahkan rujukan ke sumber tepercaya. Pernyataan tak bersumber bisa saja dipertentangkan dan dihapus.Cari sumber: Injil Tomas – berita · surat kabar · buku · cendekiawan · JSTOR (Maret 2010) Bagian dari seri tentang Gnostisisme Gnostisisme Persia Mandaeisme Manikheisme Gnostisisme Suriah-Mesir Setian Tomasin Valentinian Bas…

Village in Fond du Lac and Washington counties, Wisconsin This article is about the village. For the adjacent town, see Kewaskum (town), Wisconsin. Village in Wisconsin, United StatesKewaskum, WisconsinVillageIntersection of US 45 and WIS 28 in downtown KewaskumLocation of Kewaskum in Fond du Lac County (top) and Washington County (bottom), Wisconsin.Coordinates: 43°30′51″N 88°13′24″W / 43.51417°N 88.22333°W / 43.51417; -88.22333Country United StatesState…

Demolished pier originally located at Jordan Road, Jordan, Hong Kong Jordan Road Ferry Pier c. 1964 Concourse of the Jordan Road vehicular ferry in the 1930s Bus terminus at concourse of the Jordan Road vehicular ferry in the 1950s Jordan Road Ferry Pier or Ferry Point (1924–1998) (Chinese: 佐敦道碼頭) is a demolished pier originally located at Jordan Road, Jordan, Hong Kong. History After Hongkong and Yaumati Ferry gained the franchise to operate part of the cross harbour ferry route…

British politician and peer His GraceThe Duke of RichmondKG GCVO CBPhotograph of Lord Richmond, 1907Member of Parliament for ChichesterIn office1885–1889Preceded byLord Henry LennoxJohn Abel SmithSucceeded byLord Walter Gordon-LennoxMember of Parliament for West SussexIn office1869–1885Serving with Sir Walter Barttelot, BtPreceded byHon. Henry WyndhamSir Walter Barttelot, BtSucceeded byConstituency divided Personal detailsBornCharles Henry Gordon-Lennox(1845-12-27)27 December 1845Por…
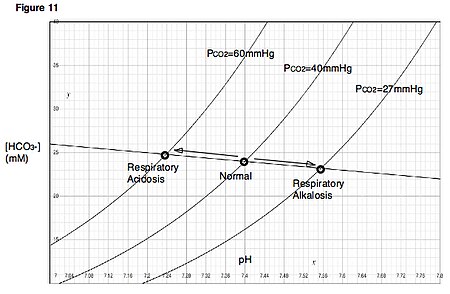
Medical condition Medical conditionRespiratory acidosisDavenport diagramSpecialtyIntensive care medicine, pulmonology, internal medicine Respiratory acidosis is a state in which decreased ventilation (hypoventilation) increases the concentration of carbon dioxide in the blood and decreases the blood's pH (a condition generally called acidosis). Carbon dioxide is produced continuously as the body's cells respire, and this CO2 will accumulate rapidly if the lungs do not adequately expel it t…

† Большая гавайская древесница Научная классификация Домен:ЭукариотыЦарство:ЖивотныеПодцарство:ЭуметазоиБез ранга:Двусторонне-симметричныеБез ранга:ВторичноротыеТип:ХордовыеПодтип:ПозвоночныеИнфратип:ЧелюстноротыеНадкласс:ЧетвероногиеКлада:АмниотыКлада:Завр…

1925 novel Colonel Gore’s Second Case 1928 editionAuthorLynn BrockLanguageEnglishSeriesColonel GoreGenreDetectivePublisherWilliam Collins, Sons Harper & Brothers (US)Publication date1925Publication placeUnited KingdomMedia typePrintPreceded byThe Deductions of Colonel Gore Followed byThe Kink Colonel Gore’s Second Case is a 1925 detective novel by the Irish writer Lynn Brock.[1] It was the second in his series of seven novels featuring the character of C…

Part of a series onChristianity JesusChrist Nativity Baptism Ministry Crucifixion Resurrection Ascension BibleFoundations Old Testament New Testament Gospel Canon Church Creed New Covenant Theology God Trinity Father Son Holy Spirit Apologetics Baptism Christology History of theology Mission Salvation Universalism HistoryTradition Apostles Peter Paul Mary Early Christianity Church Fathers Constantine Councils Augustine Ignatius East–West Schism Crusades Aquinas Reformation Luther Denominations…

American professional wrestler (born 1978) Tamina redirects here. For the river, see Tamina (river). Tamina SnukaTamina in 2016Birth nameSarona Moana Marie Reiher Snuka[1]Born (1978-01-10) January 10, 1978 (age 46)[2]Vancouver, Washington, U.S.[3]Spouse(s) Brandon Polamalu (m. 1995; div. 2003)Children2FamilyJimmy Snuka (father)Deuce (brother)Anoaʻi[4]Professional wrestling careerRing name(s)Sarona SnukaTami…

Official Secretary to the Governor-General of AustraliaIncumbentPaul Singer MVOsince 18 August 2018 (2018-08-18)Office of the Official Secretary to the Governor-GeneralAppointerGovernor-General-in-CouncilTerm lengthat the governor's pleasureInaugural holderSir George StewardFormation1903 (1903) The official secretary to the governor-general of Australia and staff provide support to the governor-general of Australia to enable the governor-general to carry out their consti…

Lili ElbeLili Elbe vào năm 1926SinhEinar Magnus Andreas Wegener(1882-12-28)28 tháng 12 năm 1882Vejle, Đan MạchMất13 tháng 9 năm 1931(1931-09-13) (48 tuổi)Dresden, ĐứcTên khácLili Ilse Elvenes Lili Ilse Elvenes, được biết đến rộng rãi với cái tên Lili Elbe (28 tháng 12 năm 1882 – 13 tháng 9 năm 1931), là một người Đan Mạch chuyển giới từ nam sang nữ. Cô là một trong những trường hợp chuyển đổi giới tính đ�…

American timeshare company This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed.Find sources: Hilton Grand Vacations – news · newspapers · books · scholar · JSTOR (July 2017) (Learn how and when to remove this message) For the former parent company, see Hilton Worldwide. Hilton Grand Vacations Inc.Company typePublic companyTraded…

Lil Wayne Dwayne Michael Carter, Jr. (lahir 27 September 1982) atau dikenal dengan Lil Wayne merupakan seorang penyanyi dan rapper berkebangsaan Amerika Serikat. Album pertamanya adalah Tha Block Is Hot dirilis pada tahun 1999. Dia dilahirkan di New Orleans, Louisiana. Dia berkarier di dunia musik sejak tahun 1997. Diskografi 1999: Tha Block Is Hot 2000: Lights Out 2002: 500 Degreez 2004: Tha Carter 2005: Tha Carter II 2008: Tha Carter III 2010: Rebirth 2010: I Am Not a Human Being 2011: Tha Car…

English footballer (born 1973) David Unsworth Unsworth as Everton caretaker manager in 2017Personal informationFull name David Gerald Unsworth[1]Date of birth (1973-10-16) 16 October 1973 (age 50)[2]Place of birth Chorley, EnglandHeight 6 ft 1 in (1.85 m)[2]Position(s) Centre-back, left-backYouth career0000–1992 EvertonSenior career*Years Team Apps (Gls)1992–1997 Everton 116 (11)1997–1998 West Ham United 32 (2)1998 Aston Villa 0 (0)1998–2004 Ev…

2011 video gameCaptain America: Super SoldierNorth American cover artDeveloper(s)Next Level GamesHigh Voltage Software (Wii/3DS)Griptonite Games (DS)Publisher(s)SegaDirector(s)Brandon GillProducer(s)Edoardo De MartinWilliam KingPaul MartinBjorn NashTania PoulterDesigner(s)Jeff King, Ian ChristyKenneth Bowen, G. Kelly Toyama, Dylan Kelly (NDS)Programmer(s)Darwin Chau, Travis Brown-JohnZak Arntson (NDS)Artist(s)Barret ChapmanJames Lutz (NDS)Writer(s)Christos Gage (story)(also NDS w Lester Milton)B…



