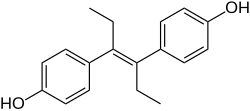
Class of drugs
A nonsteroidal estrogen is an estrogen with a nonsteroidal chemical structure .[ 1] stilbestrol estrogen diethylstilbestrol (DES).[ 1] [ 2] toxicities associated with high-dose DES starting in the early 1970s, and are now almost never used.[ 2] [ 3] [ 4] selective estrogen receptor modulators (SERMs) are nonsteroidal, with triphenylethylenes like tamoxifen and clomifene having been derived from DES,[ 5] breast cancer among other indications.[ 6] pharmaceutical drugs , many xenoestrogens , including phytoestrogens , mycoestrogens , and synthetic endocrine disruptors like bisphenol A , are nonsteroidal substances with estrogenic activity.[ 7]
Pharmacology
Nonsteroidal estrogens act as agonists of the estrogen receptors , ERα and ERβ .
Affinities of estrogen receptor ligands for the ERα and ERβ
Ligand Other names
Relative binding affinities (RBA, %)a Absolute binding affinities (Ki , nM)a Action
ERα ERβ ERα ERβ
Estradiol E2; 17β-Estradiol
100
100
0.115 (0.04–0.24)
0.15 (0.10–2.08)
Estrogen
Estrone E1; 17-Ketoestradiol
16.39 (0.7–60)
6.5 (1.36–52)
0.445 (0.3–1.01)
1.75 (0.35–9.24)
Estrogen
Estriol E3; 16α-OH-17β-E2
12.65 (4.03–56)
26 (14.0–44.6)
0.45 (0.35–1.4)
0.7 (0.63–0.7)
Estrogen
Estetrol E4; 15α,16α-Di-OH-17β-E2
4.0
3.0
4.9
19
Estrogen
Alfatradiol 17α-Estradiol
20.5 (7–80.1)
8.195 (2–42)
0.2–0.52
0.43–1.2
Metabolite
16-Epiestriol 16β-Hydroxy-17β-estradiol
7.795 (4.94–63)
50
?
?
Metabolite
17-Epiestriol 16α-Hydroxy-17α-estradiol
55.45 (29–103)
79–80
?
?
Metabolite
16,17-Epiestriol 16β-Hydroxy-17α-estradiol
1.0
13
?
?
Metabolite
2-Hydroxyestradiol 2-OH-E2
22 (7–81)
11–35
2.5
1.3
Metabolite
2-Methoxyestradiol 2-MeO-E2
0.0027–2.0
1.0
?
?
Metabolite
4-Hydroxyestradiol 4-OH-E2
13 (8–70)
7–56
1.0
1.9
Metabolite
4-Methoxyestradiol 4-MeO-E2
2.0
1.0
?
?
Metabolite
2-Hydroxyestrone 2-OH-E1
2.0–4.0
0.2–0.4
?
?
Metabolite
2-Methoxyestrone 2-MeO-E1
<0.001–<1
<1
?
?
Metabolite
4-Hydroxyestrone 4-OH-E1
1.0–2.0
1.0
?
?
Metabolite
4-Methoxyestrone 4-MeO-E1
<1
<1
?
?
Metabolite
16α-Hydroxyestrone 16α-OH-E1; 17-Ketoestriol
2.0–6.5
35
?
?
Metabolite
2-Hydroxyestriol 2-OH-E3
2.0
1.0
?
?
Metabolite
4-Methoxyestriol 4-MeO-E3
1.0
1.0
?
?
Metabolite
Estradiol sulfate E2S; Estradiol 3-sulfate
<1
<1
?
?
Metabolite
Estradiol disulfate Estradiol 3,17β-disulfate
0.0004
?
?
?
Metabolite
Estradiol 3-glucuronide E2-3G
0.0079
?
?
?
Metabolite
Estradiol 17β-glucuronide E2-17G
0.0015
?
?
?
Metabolite
Estradiol 3-gluc. 17β-sulfate E2-3G-17S
0.0001
?
?
?
Metabolite
Estrone sulfate E1S; Estrone 3-sulfate
<1
<1
>10
>10
Metabolite
Estradiol benzoate EB; Estradiol 3-benzoate
10
?
?
?
Estrogen
Estradiol 17β-benzoate E2-17B
11.3
32.6
?
?
Estrogen
Estrone methyl ether Estrone 3-methyl ether
0.145
?
?
?
Estrogen
ent -Estradiol1-Estradiol
1.31–12.34
9.44–80.07
?
?
Estrogen
Equilin 7-Dehydroestrone
13 (4.0–28.9)
13.0–49
0.79
0.36
Estrogen
Equilenin 6,8-Didehydroestrone
2.0–15
7.0–20
0.64
0.62
Estrogen
17β-Dihydroequilin 7-Dehydro-17β-estradiol
7.9–113
7.9–108
0.09
0.17
Estrogen
17α-Dihydroequilin 7-Dehydro-17α-estradiol
18.6 (18–41)
14–32
0.24
0.57
Estrogen
17β-Dihydroequilenin 6,8-Didehydro-17β-estradiol
35–68
90–100
0.15
0.20
Estrogen
17α-Dihydroequilenin 6,8-Didehydro-17α-estradiol
20
49
0.50
0.37
Estrogen
Δ8 -Estradiol 8,9-Dehydro-17β-estradiol
68
72
0.15
0.25
Estrogen
Δ8 -Estrone 8,9-Dehydroestrone
19
32
0.52
0.57
Estrogen
Ethinylestradiol EE; 17α-Ethynyl-17β-E2
120.9 (68.8–480)
44.4 (2.0–144)
0.02–0.05
0.29–0.81
Estrogen
Mestranol EE 3-methyl ether
?
2.5
?
?
Estrogen
Moxestrol RU-2858; 11β-Methoxy-EE
35–43
5–20
0.5
2.6
Estrogen
Methylestradiol 17α-Methyl-17β-estradiol
70
44
?
?
Estrogen
Diethylstilbestrol DES; Stilbestrol
129.5 (89.1–468)
219.63 (61.2–295)
0.04
0.05
Estrogen
Hexestrol Dihydrodiethylstilbestrol
153.6 (31–302)
60–234
0.06
0.06
Estrogen
Dienestrol Dehydrostilbestrol
37 (20.4–223)
56–404
0.05
0.03
Estrogen
Benzestrol (B2) –
114
?
?
?
Estrogen
Chlorotrianisene TACE
1.74
?
15.30
?
Estrogen
Triphenylethylene TPE
0.074
?
?
?
Estrogen
Triphenylbromoethylene TPBE
2.69
?
?
?
Estrogen
Tamoxifen ICI-46,474
3 (0.1–47)
3.33 (0.28–6)
3.4–9.69
2.5
SERM
Afimoxifene 4-Hydroxytamoxifen; 4-OHT
100.1 (1.7–257)
10 (0.98–339)
2.3 (0.1–3.61)
0.04–4.8
SERM
Toremifene 4-Chlorotamoxifen; 4-CT
?
?
7.14–20.3
15.4
SERM
Clomifene MRL-41
25 (19.2–37.2)
12
0.9
1.2
SERM
Cyclofenil F-6066; Sexovid
151–152
243
?
?
SERM
Nafoxidine U-11,000A
30.9–44
16
0.3
0.8
SERM
Raloxifene –
41.2 (7.8–69)
5.34 (0.54–16)
0.188–0.52
20.2
SERM
Arzoxifene LY-353,381
?
?
0.179
?
SERM
Lasofoxifene CP-336,156
10.2–166
19.0
0.229
?
SERM
Ormeloxifene Centchroman
?
?
0.313
?
SERM
Levormeloxifene 6720-CDRI; NNC-460,020
1.55
1.88
?
?
SERM
Ospemifene Deaminohydroxytoremifene
0.82–2.63
0.59–1.22
?
?
SERM
Bazedoxifene –
?
?
0.053
?
SERM
Etacstil GW-5638
4.30
11.5
?
?
SERM
ICI-164,384 –
63.5 (3.70–97.7)
166
0.2
0.08
Antiestrogen
Fulvestrant ICI-182,780
43.5 (9.4–325)
21.65 (2.05–40.5)
0.42
1.3
Antiestrogen
Propylpyrazoletriol PPT
49 (10.0–89.1)
0.12
0.40
92.8
ERα agonist
16α-LE2 16α-Lactone-17β-estradiol
14.6–57
0.089
0.27
131
ERα agonist
16α-Iodo-E2 16α-Iodo-17β-estradiol
30.2
2.30
?
?
ERα agonist
Methylpiperidinopyrazole MPP
11
0.05
?
?
ERα antagonist
Diarylpropionitrile DPN
0.12–0.25
6.6–18
32.4
1.7
ERβ agonist
8β-VE2 8β-Vinyl-17β-estradiol
0.35
22.0–83
12.9
0.50
ERβ agonist
Prinaberel ERB-041; WAY-202,041
0.27
67–72
?
?
ERβ agonist
ERB-196 WAY-202,196
?
180
?
?
ERβ agonist
Erteberel SERBA-1; LY-500,307
?
?
2.68
0.19
ERβ agonist
SERBA-2 –
?
?
14.5
1.54
ERβ agonist
Coumestrol –
9.225 (0.0117–94)
64.125 (0.41–185)
0.14–80.0
0.07–27.0
Xenoestrogen
Genistein –
0.445 (0.0012–16)
33.42 (0.86–87)
2.6–126
0.3–12.8
Xenoestrogen
Equol –
0.2–0.287
0.85 (0.10–2.85)
?
?
Xenoestrogen
Daidzein –
0.07 (0.0018–9.3)
0.7865 (0.04–17.1)
2.0
85.3
Xenoestrogen
Biochanin A –
0.04 (0.022–0.15)
0.6225 (0.010–1.2)
174
8.9
Xenoestrogen
Kaempferol –
0.07 (0.029–0.10)
2.2 (0.002–3.00)
?
?
Xenoestrogen
Naringenin –
0.0054 (<0.001–0.01)
0.15 (0.11–0.33)
?
?
Xenoestrogen
8-Prenylnaringenin 8-PN
4.4
?
?
?
Xenoestrogen
Quercetin –
<0.001–0.01
0.002–0.040
?
?
Xenoestrogen
Ipriflavone –
<0.01
<0.01
?
?
Xenoestrogen
Miroestrol –
0.39
?
?
?
Xenoestrogen
Deoxymiroestrol –
2.0
?
?
?
Xenoestrogen
β-Sitosterol –
<0.001–0.0875
<0.001–0.016
?
?
Xenoestrogen
Resveratrol –
<0.001–0.0032
?
?
?
Xenoestrogen
α-Zearalenol –
48 (13–52.5)
?
?
?
Xenoestrogen
β-Zearalenol –
0.6 (0.032–13)
?
?
?
Xenoestrogen
Zeranol α-Zearalanol
48–111
?
?
?
Xenoestrogen
Taleranol β-Zearalanol
16 (13–17.8)
14
0.8
0.9
Xenoestrogen
Zearalenone ZEN
7.68 (2.04–28)
9.45 (2.43–31.5)
?
?
Xenoestrogen
Zearalanone ZAN
0.51
?
?
?
Xenoestrogen
Bisphenol A BPA
0.0315 (0.008–1.0)
0.135 (0.002–4.23)
195
35
Xenoestrogen
Endosulfan EDS
<0.001–<0.01
<0.01
?
?
Xenoestrogen
Kepone Chlordecone
0.0069–0.2
?
?
?
Xenoestrogen
o,p' -DDT–
0.0073–0.4
?
?
?
Xenoestrogen
p,p' -DDT–
0.03
?
?
?
Xenoestrogen
Methoxychlor p,p' -Dimethoxy-DDT0.01 (<0.001–0.02)
0.01–0.13
?
?
Xenoestrogen
HPTE Hydroxychlor; p,p' -OH-DDT
1.2–1.7
?
?
?
Xenoestrogen
Testosterone T; 4-Androstenolone
<0.0001–<0.01
<0.002–0.040
>5000
>5000
Androgen
Dihydrotestosterone DHT; 5α-Androstanolone
0.01 (<0.001–0.05)
0.0059–0.17
221–>5000
73–1688
Androgen
Nandrolone 19-Nortestosterone; 19-NT
0.01
0.23
765
53
Androgen
Dehydroepiandrosterone DHEA; Prasterone
0.038 (<0.001–0.04)
0.019–0.07
245–1053
163–515
Androgen
5-Androstenediol A5; Androstenediol
6
17
3.6
0.9
Androgen
4-Androstenediol –
0.5
0.6
23
19
Androgen
4-Androstenedione A4; Androstenedione
<0.01
<0.01
>10000
>10000
Androgen
3α-Androstanediol 3α-Adiol
0.07
0.3
260
48
Androgen
3β-Androstanediol 3β-Adiol
3
7
6
2
Androgen
Androstanedione 5α-Androstanedione
<0.01
<0.01
>10000
>10000
Androgen
Etiocholanedione 5β-Androstanedione
<0.01
<0.01
>10000
>10000
Androgen
Methyltestosterone 17α-Methyltestosterone
<0.0001
?
?
?
Androgen
Ethinyl-3α-androstanediol 17α-Ethynyl-3α-adiol
4.0
<0.07
?
?
Estrogen
Ethinyl-3β-androstanediol 17α-Ethynyl-3β-adiol
50
5.6
?
?
Estrogen
Progesterone P4; 4-Pregnenedione
<0.001–0.6
<0.001–0.010
?
?
Progestogen
Norethisterone NET; 17α-Ethynyl-19-NT
0.085 (0.0015–<0.1)
0.1 (0.01–0.3)
152
1084
Progestogen
Norethynodrel 5(10)-Norethisterone
0.5 (0.3–0.7)
<0.1–0.22
14
53
Progestogen
Tibolone 7α-Methylnorethynodrel
0.5 (0.45–2.0)
0.2–0.076
?
?
Progestogen
Δ4 -Tibolone 7α-Methylnorethisterone
0.069–<0.1
0.027–<0.1
?
?
Progestogen
3α-Hydroxytibolone –
2.5 (1.06–5.0)
0.6–0.8
?
?
Progestogen
3β-Hydroxytibolone –
1.6 (0.75–1.9)
0.070–0.1
?
?
Progestogen
Footnotes: a = (1) Binding affinity values are of the format "median (range)" (# (#–#)), "range" (#–#), or "value" (#) depending on the values available. The full sets of values within the ranges can be found in the Wiki code. (2) Binding affinities were determined via displacement studies in a variety of in-vitro labeled estradiol and human ERα and ERβ proteins (except the ERβ values from Kuiper et al. (1997), which are rat ERβ). Sources: See template page.
List of nonsteroidal estrogens
Synthetic
Pharmaceutical
Stilbestrols : benzestrol , bifluranol , dienestrol , diethylstilbestrol , dimestrol , fosfestrol , furostilbestrol , hexestrol , mestilbol , methestrol , pentafluranol , phenestrol , terfluranol , stilbestrol esters Triphenylethylenes : chlorotrianisene , desmethylchlorotrianisene , estrobin (DBE ) , M2613 , triphenylbromoethylene , triphenylchloroethylene , triphenyliodoethylene , triphenylmethylethylene Secosteroids (open-ring steroids): allenestrol , allenolic acid , bisdehydrodoisynolic acid , carbestrol , doisynoestrol , doisynolic acid , fenestrel , methallenestril Selective ERα or ERβ agonists : diarylpropionitrile , ERB-196 , erteberel , FERb 033 , GTx-758 , prinaberel , propylpyrazoletriol , WAY-166818 , WAY-214156 Others: 2,8-dihydroxyhexahydrochrysene (2,8-DHHHC), paroxypropione , quadrosilan , tetrahydrochrysene SERMs like tamoxifen and raloxifene can also be considered to be nonsteroidal estrogens in some tissues .[ 8]
Environmental
Natural
Metalloestrogens : cadmium , othersMycoestrogens : taleranol (β-zearalanol), α-zearalenol , β-zearalenol , zearalanone , zearalenone , zeranol (α-zearalanol)Phytoestrogens : coumestrol , daidzein , deoxymiroestrol , equol , genistein , miroestrol , many others
See also
References
Further reading
Hermkens PH, Kamp S, Lusher S, Veeneman GH (2006). "Non-steroidal steroid receptor modulators". IDrugs . 9 (7): 488–94. doi :10.2174/0929867053764671 . PMID 16821162 . Mohler ML, Narayanan R, Coss CC, Hu K, He Y, Wu Z, Hong SS, Hwang DJ, Miller DD, Dalton JT (2010). "Estrogen receptor beta selective nonsteroidal estrogens: seeking clinical indications". Expert Opin Ther Pat . 20 (4): 507–34. doi :10.1517/13543771003657164 . PMID 20302450 . S2CID 314347 .
Estrogens
ER Tooltip Estrogen receptor agonists
Steroidal: Alfatradiol Certain androgens /anabolic steroids (e.g., testosterone , testosterone esters , methyltestosterone , metandienone , nandrolone esters ) (via estrogenic metabolites)
Certain progestins (e.g., norethisterone , noretynodrel , etynodiol diacetate , tibolone )
Clomestrone Cloxestradiol acetate Conjugated estriol Conjugated estrogens Epiestriol Epimestrol Esterified estrogens Estetrol † Estradiol Estradiol esters (e.g., estradiol acetate , estradiol benzoate , estradiol cypionate , estradiol enanthate , estradiol undecylate , estradiol valerate , polyestradiol phosphate , estradiol ester mixtures (Climacteron ))Estramustine phosphate Estriol Estriol esters (e.g., estriol succinate , polyestriol phosphate )Estrogenic substances Estrone Estrone esters
Ethinylestradiol #
Hydroxyestrone diacetate Mestranol Methylestradiol Moxestrol Nilestriol Prasterone (dehydroepiandrosterone; DHEA)
Promestriene Quinestradol Quinestrol Progonadotropins
Antiestrogens
ER Tooltip Estrogen receptor antagonistsSERMs Tooltip selective estrogen receptor modulators /SERDs Tooltip selective estrogen receptor downregulators )Aromatase inhibitors Antigonadotropins
Androgens /anabolic steroids (e.g., testosterone , testosterone esters , nandrolone esters , oxandrolone , fluoxymesterone )D2 receptor antagonists (prolactin releasers) (e.g., domperidone , metoclopramide , risperidone , haloperidol , chlorpromazine , sulpiride )GnRH agonistsleuprorelin , goserelin )GnRH antagonistscetrorelix , elagolix )Progestogens (e.g., chlormadinone acetate , cyproterone acetate , gestonorone caproate , hydroxyprogesterone caproate , medroxyprogesterone acetate , megestrol acetate ) Others
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown