Chemical compound
Pharmaceutical compound
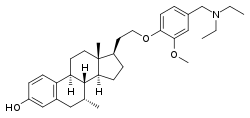
TAS-108 Other names 17β-[2-[4-[(diethylamino)methyl]-2-methoxyphenoxy]ethyl]-7α-methylestra-1,3,5(10)-trien-3-ol; 17β-[2-[4-[(diethylamino)methyl]-2-methoxyphenoxy]ethyl]-7α-methylestradiol Routes of By mouth [ 1] ATC code
(1S,9R,10S,11S,14R,15R)-14-(2-{4-[(diethylamino)methyl]-2-methoxyphenoxy}ethyl)-9,15-dimethyltetracyclo[8.7.0.02,7 .011,15 ]heptadeca-2(7),3,5-trien-5-ol
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 33 H 47 N O 3 Molar mass −1 3D model (JSmol )
CCN(CC)CC1=CC(=C(C=C1)OCC[C@H]2CC[C@@H]3[C@@]2(CC[C@H]4[C@H]3[C@@H](CC5=C4C=CC(=C5)O)C)C)OC
Citrate: CCN(CC)CC1=CC(=C(C=C1)OCC[C@H]2CC[C@@H]3[C@@]2(CC[C@H]4[C@H]3[C@@H](CC5=C4C=CC(=C5)O)C)C)OC.C(C(=O)O)C(CC(=O)O)(C(=O)O)O
InChI=1S/C33H47NO3/c1-6-34(7-2)21-23-8-13-30(31(19-23)36-5)37-17-15-25-9-12-29-32-22(3)18-24-20-26(35)10-11-27(24)28(32)14-16-33(25,29)4/h8,10-11,13,19-20,22,25,28-29,32,35H,6-7,9,12,14-18,21H2,1-5H3/t22-,25-,28-,29+,32-,33-/m1/s1
Y Key:OHCPNHFLPCVWRG-MWSJHZLTSA-N
Y Citrate: InChI=1S/C33H47NO3.C6H8O7/c1-6-34(7-2)21-23-8-13-30(31(19-23)36-5)37-17-15-25-9-12-29-32-22(3)18-24-20-26(35)10-11-27(24)28(32)14-16-33(25,29)4;7-3(8)1-6(13,5(11)12)2-4(9)10/h8,10-11,13,19-20,22,25,28-29,32,35H,6-7,9,12,14-18,21H2,1-5H3;13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)/t22-,25-,28-,29+,32-,33-;/m1./s1
Key:VOHOCSJONOJOSD-SCIDSJFVSA-N
TAS-108 , also known as SR-16234 , is a drug discovered by Masato Tanabe and under development by SRI International and Taiho Pharmaceutical . It is a steroid hormone that has shown signs of treating and preventing breast cancer , even in patients where tamoxifen has failed.[ 2] [ 3]
Development
Masato Tanabe's team at SRI has focused on the development of steroid hormones. A compound discovered in a previous SRI contract from the National Institutes of Health showed potential – it acted like "anti-estrogen" in the breasts and uterus but like normal estrogen elsewhere in the body, and was more "tissue-selective".[ 4] Taiho Pharmaceutical in July 1996, and within six years and slightly under $3 million (an unusually short amount of time), two new drugs were discovered and tested on people (particularly people for which tamoxifen has failed): SR-16234 and SR-16287.[ 4]
The first of those, SR-16234, also inhibited the growth of blood vessels angiogenesis and accelerated the death of cancer cells apoptosis and thus was particularly well suited to be an anti-cancer drug.[ 4] [ 5] [ 6]
See also
References
^ Yamamoto Y, Shibata J, Yonekura K, Sato K, Hashimoto A, Aoyagi Y, et al. (January 2005). "TAS-108, a novel oral steroidal antiestrogenic agent, is a pure antagonist on estrogen receptor alpha and a partial agonist on estrogen receptor beta with low uterotrophic effect". Clinical Cancer Research . 11 (1): 315– 322. doi :10.1158/1078-0432.315.11.1 . PMID 15671561 . ^ Yamamoto Y, Wada O, Takada I, Yogiashi Y, Shibata J, Yanagisawa J, et al. (December 2003). "Both N- and C-terminal transactivation functions of DNA-bound ERalpha are blocked by a novel synthetic estrogen ligand". Biochemical and Biophysical Research Communications . 312 (3): 656– 662. doi :10.1016/j.bbrc.2003.10.178 . PMID 14680815 . ^ "Alumni Hall of Fame 2004: Masato Tanabe" . SRI International . Retrieved 2013-02-10 .^ a b c Nielson D (2006). A Heritage of Innovation: SRI's First Half Century . Menlo Park, California : SRI International. pp. 10– 15. ISBN 978-0-9745208-1-0 ^ "SRI International to Advance Clinical Development of TAS-108, a Late-Stage Breast Cancer Drug" (Press release). SRI International . Retrieved 2013-02-24 .^ Buzdar AU (January 2005). "TAS-108: a novel steroidal antiestrogen" . Clinical Cancer Research . 11 (2 Pt 2): 906s – 908s . doi :10.1158/1078-0432.906s.11.2 PMID 15701885 .
External links
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown