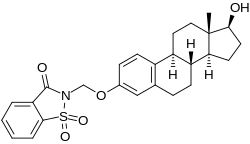
Estradiol 3-saccharinylmethyl ether Chemical compound
Pharmaceutical compound
Estradiol 3-saccharinylmethyl ether Other names E2SME; 3-O -(Saccharinylmethyl)-17β-estradiol; 3-O -(Saccharinylmethyl)estra-1,3,5(10)-triene-3,17β-diol Routes of By mouth [ 1] Drug class Estrogen
2-[[(8R ,9S ,13S ,14S ,17S )-17-hydroxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a ]phenanthren-3-yl]oxymethyl]-1,1-dioxo-1,2-benzothiazol-3-one
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 26 H 29 N O 5 S Molar mass −1 3D model (JSmol )
C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)CCC4=C3C=CC(=C4)OCN5C(=O)C6=CC=CC=C6S5(=O)=O
InChI=1S/C26H29NO5S/c1-26-13-12-19-18-9-7-17(14-16(18)6-8-20(19)22(26)10-11-24(26)28)32-15-27-25(29)21-4-2-3-5-23(21)33(27,30)31/h2-5,7,9,14,19-20,22,24,28H,6,8,10-13,15H2,1H3/t19-,20-,22+,24+,26+/m1/s1
Key:QNUCLUWOICXPIY-QETBJLDASA-N
Estradiol 3-saccharinylmethyl ether (E2SME ), also known as 3-O -(saccharinylmethyl)-17β-estradiol , is a synthetic estrogen and estrogen ether – specifically, the C3 saccharinyl methyl ether of estradiol – which was described in the mid-1990s and was never marketed.[ 2] [ 1] [ 3] [ 4] prodrug of estradiol and appears to be partially protected from first-pass metabolism in the liver and intestines with oral administration, showing greatly improved oral potency compared to estradiol.[ 1] [ 3] [ 4]
E2SME has been found to be 9-fold as potent as estradiol by the oral route in rats.[ 1] [ 4] bioavailability (16%) was 5-fold greater than that of estradiol via the oral route in rats, and the elimination half-life of released estradiol was 5- to 7-fold longer than that of regular estradiol.[ 1] [ 3] [ 4] intravenously in rats, there was no difference between them in terms of potency.[ 1] In vitro hydrolyzed to estradiol enzymatically but rather is hydrolyzed chemically in biological media such as plasma , apparently dependent on the concentration of protein .[ 1]
See also
References
^ a b c d e f g Patel J, Katovich MJ, Sloan KB, Curry SH, Prankerd RJ (February 1995). "A prodrug approach to increasing the oral potency of a phenolic drug. Part 2. Pharmacodynamics and preliminary bioavailability of an orally administered O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences . 84 (2): 174– 178. doi :10.1002/jps.2600840210 . PMID 7738796 . ^ Patel JU, Prankerd RJ, Sloan KB (October 1994). "A prodrug approach to increasing the oral potency of a phenolic drug. 1. Synthesis, characterization, and stability of an O-(imidomethyl) derivative of 17 beta-estradiol". Journal of Pharmaceutical Sciences . 83 (10): 1477– 1481. doi :10.1002/jps.2600831022 . PMID 7884673 . ^ a b c Kuhnz W, Blode H, Zimmerman H (6 December 2012). "Pharmacokinetics of Exogenous Natural and Synthetic Estrogens and Antiestrogens" . In Oettel M, Schillinger E (eds.). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen . Springer Science & Business Media. pp. 263–. ISBN 978-3-642-60107-1 ^ a b c d Aungst BJ, Matz N (2007). "Prodrugs to Reduce Presystemic Metabolism". Prodrugs . Biotechnology: Pharmaceutical Aspects. Vol. V. Springer. pp. 339– 355. doi :10.1007/978-0-387-49785-3_8 . ISBN 978-0-387-49782-2
ER Tooltip Estrogen receptor
Agonists
Steroidal: 2-Hydroxyestradiol 2-Hydroxyestrone 3-Methyl-19-methyleneandrosta-3,5-dien-17β-ol 3α-Androstanediol 3α,5α-Dihydrolevonorgestrel 3β,5α-Dihydrolevonorgestrel 3α-Hydroxytibolone 3β-Hydroxytibolone 3β-Androstanediol 4-Androstenediol 4-Androstenedione 4-Fluoroestradiol 4-Hydroxyestradiol 4-Hydroxyestrone 4-Methoxyestradiol 4-Methoxyestrone 5-Androstenediol 7-Oxo-DHEA 7α-Hydroxy-DHEA 7α-Methylestradiol 7β-Hydroxyepiandrosterone 8,9-Dehydroestradiol 8,9-Dehydroestrone 8β-VE2 10β,17β-Dihydroxyestra-1,4-dien-3-one (DHED) 11β-Chloromethylestradiol 11β-Methoxyestradiol 15α-Hydroxyestradiol 16-Ketoestradiol 16-Ketoestrone 16α-Fluoroestradiol 16α-Hydroxy-DHEA 16α-Hydroxyestrone 16α-Iodoestradiol 16α-LE2 16β-Hydroxyestrone 16β,17α-Epiestriol (16β-hydroxy-17α-estradiol) 17α-Estradiol (alfatradiol )17α-Dihydroequilenin 17α-Dihydroequilin 17α-Epiestriol (16α-hydroxy-17α-estradiol) 17α-Ethynyl-3α-androstanediol 17α-Ethynyl-3β-androstanediol 17β-Dihydroequilenin 17β-Dihydroequilin 17β-Methyl-17α-dihydroequilenin Abiraterone Abiraterone acetate Alestramustine Almestrone Anabolic steroids (e.g., testosterone and esters , methyltestosterone , metandienone (methandrostenolone) , nandrolone and esters , many others; via estrogenic metabolites)Atrimustine Bolandiol Bolandiol dipropionate Butolame Clomestrone Cloxestradiol
Conjugated estriol Conjugated estrogens Cyclodiol Cyclotriol DHEA DHEA-S ent -EstradiolEpiestriol (16β-epiestriol, 16β-hydroxy-17β-estradiol) Epimestrol Equilenin Equilin ERA-63 (ORG-37663) Esterified estrogens Estetrol Estradiol
Estramustine Estramustine phosphate Estrapronicate Estrazinol Estriol
Estrofurate Estrogenic substances Estromustine Estrone
Etamestrol (eptamestrol) Ethinylandrostenediol
Ethinylestradiol
Ethinylestriol Ethylestradiol Etynodiol Etynodiol diacetate Hexolame Hippulin Hydroxyestrone diacetate Lynestrenol Lynestrenol phenylpropionate Mestranol Methylestradiol Moxestrol Mytatrienediol Nilestriol Norethisterone Noretynodrel Orestrate Pentolame Prodiame Prolame Promestriene RU-16117 Quinestradol Quinestrol Tibolone Xenoestrogens: Anise -related (e.g., anethole , anol , dianethole , dianol , photoanethole )Chalconoids (e.g., isoliquiritigenin , phloretin , phlorizin (phloridzin) , wedelolactone )Coumestans (e.g., coumestrol , psoralidin )Flavonoids (incl. 7,8-DHF , 8-prenylnaringenin , apigenin , baicalein , baicalin , biochanin A , calycosin , catechin , daidzein , daidzin , ECG , EGCG , epicatechin , equol , formononetin , glabrene , glabridin , genistein , genistin , glycitein , kaempferol , liquiritigenin , mirificin , myricetin , naringenin , penduletin , pinocembrin , prunetin , puerarin , quercetin , tectoridin , tectorigenin )Lavender oil Lignans (e.g., enterodiol , enterolactone , nyasol (cis -hinokiresinol) )Metalloestrogens (e.g., cadmium )Pesticides (e.g., alternariol , dieldrin , endosulfan , fenarimol , HPTE , methiocarb , methoxychlor , triclocarban , triclosan )Phytosteroids (e.g., digitoxin (digitalis ), diosgenin , guggulsterone )Phytosterols (e.g., β-sitosterol , campesterol , stigmasterol )Resorcylic acid lactones (e.g., zearalanone , α-zearalenol , β-zearalenol , zearalenone , zeranol (α-zearalanol) , taleranol (teranol, β-zearalanol) )Steroid -like (e.g., deoxymiroestrol , miroestrol )Stilbenoids (e.g., resveratrol , rhaponticin )Synthetic xenoestrogens (e.g., alkylphenols , bisphenols (e.g., BPA , BPF , BPS ), DDT , parabens , PBBs , PHBA , phthalates , PCBs )Others (e.g., agnuside , rotundifuran ) MixedSERMs Tooltip Selective estrogen receptor modulators ) Antagonists
Coregulator-binding modulators: ERX-11
GPER Tooltip G protein-coupled estrogen receptor
Agonists Antagonists Unknown