|
BioprospectingMany important medications have been discovered by bioprospecting including the diabetes drug metformin (developed from a natural product found in Galega officinalis).[1] Bioprospecting (also known as biodiversity prospecting) is the exploration of natural sources for small molecules, macromolecules and biochemical and genetic information that could be developed into commercially valuable products for the agricultural,[2][3] aquaculture,[4][5] bioremediation,[4][6] cosmetics,[7][8] nanotechnology,[4][9] or pharmaceutical[2][10] industries. In the pharmaceutical industry, for example, almost one third of all small-molecule drugs approved by the U.S. Food and Drug Administration (FDA) between 1981 and 2014 were either natural products or compounds derived from natural products.[11] Terrestrial plants, fungi and actinobacteria have been the focus of many past bioprospecting programs,[12] but interest is growing in less explored ecosystems (e.g. seas and oceans) and organisms (e.g. myxobacteria, archaea) as a means of identifying new compounds with novel biological activities.[7][10][13][14] Species may be randomly screened for bioactivity or rationally selected and screened based on ecological, ethnobiological, ethnomedical, historical or genomic information.[10][15][16] When a region's biological resources or indigenous knowledge are unethically appropriated or commercially exploited without providing fair compensation, this is known as biopiracy.[12][17] Various international treaties have been negotiated to provide countries legal recourse in the event of biopiracy and to offer commercial actors legal certainty for investment. These include the UN Convention on Biological Diversity and the Nagoya Protocol.[2][10] The WIPO is currently negotiating more treaties to bridge gaps in this field. Other risks associated with bioprospecting are the overharvesting of individual species and environmental damage, but legislation has been developed to combat these also. Examples include national laws such as the US Marine Mammal Protection Act and US Endangered Species Act, and international treaties such as the UN Convention on Biological Diversity, the UN Convention on the Law of the Sea, the Biodiversity Beyond National Jurisdictions Treaty, and the Antarctic Treaty.[10][18] Bioprospecting-derived resources and productsAgriculture Bioprospecting-derived resources and products used in agriculture include biofertilizers, biopesticides and veterinary antibiotics. Rhizobium is a genus of soil bacteria used as biofertilizers,[20] Bacillus thuringiensis (also called Bt) and the annonins (obtained from seeds of the plant Annona squamosa) are examples of biopesticides,[21][22][19][23] and valnemulin and tiamulin (discovered and developed from the basidiomycete fungi Omphalina mutila and Clitopilus passeckerianus) are examples of veterinary antibiotics.[24][25] BioremediationExamples of bioprospecting products used in bioremediation include Coriolopsis gallica- and Phanerochaete chrysosporium-derived laccase enzymes, used for treating beer factory wastewater and for dechlorinating and decolorizing paper mill effluent.[9] Cosmetics and personal careCosmetics and personal care products obtained from bioprospecting include Porphyridium cruentum-derived oligosaccharide and oligoelement blends used to treat erythema (rosacea, flushing and dark circles),[7] Xanthobacter autotrophicus-derived zeaxanthin used for skin hydration and UV protection,[8] Clostridium histolyticum-derived collagenases used for skin regeneration,[8] and Microsporum-derived keratinases used for hair removal.[8] Nanotechnology and biosensorsBecause microbial laccases have a broad substrate range, they can be used in biosensor technology to detect a wide range of organic compounds. For example, laccase-containing electrodes are used to detect polyphenolic compounds in wine, and lignins and phenols in wastewater.[9] Pharmaceuticals Many of the antibacterial drugs in current clinical use were discovered through bioprospecting including the aminoglycosides, tetracyclines, amphenicols, polymyxins, cephalosporins and other β-lactam antibiotics, macrolides, pleuromutilins, glycopeptides, rifamycins, lincosamides, streptogramins, and phosphonic acid antibiotics.[10][26] The aminoglycoside antibiotic streptomycin, for example, was discovered from the soil bacterium Streptomyces griseus, the fusidane antibiotic fusidic acid was discovered from the soil fungus Acremonium fusidioides, and the pleuromutilin antibiotics (eg. lefamulin) were discovered and developed from the basidiomycete fungi Omphalina mutila and Clitopilus passeckerianus.[10][24] Other examples of bioprospecting-derived anti-infective drugs include the antifungal drug griseofulvin (discovered from the soil fungus Penicillium griseofulvum),[27] the antifungal and antileishmanial drug amphotericin B (discovered from the soil bacterium Streptomyces nodosus),[28] the antimalarial drug artemisinin (discovered from the plant Artemisia annua),[1][29] and the antihelminthic drug ivermectin (developed from the soil bacterium Streptomyces avermitilis).[30] Bioprospecting-derived pharmaceuticals have been developed for the treatment of non-communicable diseases and conditions too. These include the anticancer drug bleomycin (obtained from the soil bacterium Streptomyces verticillus),[31] the immunosuppressant drug ciclosporin used to treat autoimmune diseases such as rheumatoid arthritis and psoriasis (obtained from the soil fungus Tolypocladium inflatum),[32] the anti-inflammatory drug colchicine used to treat and prevent gout flares (obtained from the plant Colchicum autumnale),[1] the analgesic drug ziconotide (developed from the cone snail Conus magus),[13] and the acetylcholinesterase inhibitor galantamine used to treat Alzheimer's disease (obtained from plants in the Galanthus genus).[33] Bioprospecting as a discovery strategyBioprospecting has both strengths and weaknesses as a strategy for discovering new genes, molecules, and organisms suitable for development and commercialization. Strengths Bioprospecting-derived small molecules (also known as natural products) are more structurally complex than synthetic chemicals, and therefore show greater specificity towards biological targets. This is a big advantage in drug discovery and development, especially pharmacological aspects of drug discovery and development, where off-target effects can cause adverse drug reactions.[10] Natural products are also more amenable to membrane transport than synthetic compounds. This is advantageous when developing antibacterial drugs, which may need to traverse both an outer membrane and plasma membrane to reach their target.[10] For some biotechnological innovations to work, it is important to have enzymes that function at unusually high or low temperatures. An example of this is the polymerase chain reaction (PCR), which is dependent on a DNA polymerase that can operate at 60°C and above.[14] In other situations, for example dephosphorylation, it can be desirable to run the reaction at low temperature.[13] Extremophile bioprospecting is an important source of such enzymes, yielding thermostable enzymes such as Taq polymerase (from Thermus aquaticus),[14] and cold-adapted enzymes such as shrimp alkaline phosphatase (from Pandalus borealis).[13] With the Convention on Biological Diversity (CBD) now ratified by most countries, bioprospecting has the potential to bring biodiversity-rich and technologically advanced nations together, and benefit them both educationally and economically (eg. information sharing, technology transfer, new product development, royalty payment).[2][35] For useful molecules identified through microbial bioprospecting, scale up of production is feasible at reasonable cost because the producing microorganism can be cultured in a bioreactor.[8][36] Weaknesses Although some potentially very useful microorganisms are known to exist in nature (eg. lignocellulose-metabolizing microbes), difficulties have been encountered cultivating these in a laboratory setting.[38] This problem may be resolvable by genetically manipulating easier-to-culture organisms such as Escherichia coli or Streptomyces coelicolor to express the gene cluster responsible for the desired activity.[14][39] Isolating and identifying the compound(s) responsible for a biological extract's activity can be difficult.[39] Also, subsequent elucidation of the mechanism of action of the isolated compound can be time-consuming.[39] Technological advancements in liquid chromatography, mass spectrometry and other techniques are helping to overcome these challenges.[39] Implementing and enforcing bioprospecting-related treaties and legislation is not always easy.[2][35] Drug development is an inherently expensive and time-consuming process with low success rates, and this makes it difficult to quantify the value of potential products when drafting bioprospecting agreements.[2] Intellectual property rights may be difficult to award too. For example, legal rights to a medicinal plant may be disputable if it has been discovered by different people in different parts of the world at different times.[2] Whilst the structural complexity of natural products is generally advantageous in drug discovery, it can make the subsequent manufacture of drug candidates difficult. This problem is sometimes resolvable by identifying the part of the natural product structure responsible for activity and developing a simplified synthetic analogue. This was necessary with the natural product halichondrin B, its simplified analogue eribulin now approved and marketed as an anticancer drug.[40] Bioprospecting pitfallsErrors and oversights can occur at different steps in the bioprospecting process including collection of source material, screening source material for bioactivity, testing isolated compounds for toxicity, and identification of mechanism of action. Collection of source material Prior to collecting biological material or traditional knowledge, the correct permissions must be obtained from the source country, land owner etc. Failure to do so can result in criminal proceedings and rejection of any subsequent patent applications. It is also important to collect biological material in adequate quantities, to have biological material formally identified, and to deposit a voucher specimen with a repository for long-term preservation and storage. This helps ensure any important discoveries are reproducible.[10][13] Bioactivity and toxicity testingWhen testing extracts and isolated compounds for bioactivity and toxicity, the use of standard protocols (eg. CLSI, ISO, NIH, EURL ECVAM, OECD) is desirable because this improves test result accuracy and reproducibility. Also, if the source material is likely to contain known (previously discovered) active compounds (eg. streptomycin in the case of actinomycetes), then dereplication is necessary to exclude these extracts and compounds from the discovery pipeline as early as possible. In addition, it is important to consider solvent effects on the cells or cell lines being tested, to include reference compounds (ie. pure chemical compounds for which accurate bioactivity and toxicity data are available), to set limits on cell line passage number (eg. 10–20 passages), to include all the necessary positive and negative controls, and to be aware of assay limitations. These steps help ensure assay results are accurate, reproducible and interpreted correctly.[10][13] Identification of mechanism of actionWhen attempting to elucidate the mechanism of action of an extract or isolated compound, it is important to use multiple orthogonal assays. Using just a single assay, especially a single in vitro assay, gives a very incomplete picture of an extract or compound's effect on the human body.[41][42] In the case of Valeriana officinalis root extract, for example, the sleep-inducing effects of this extract are due to multiple compounds and mechanisms including interaction with GABA receptors and relaxation of smooth muscle.[41] The mechanism of action of an isolated compound can also be misidentified if a single assay is used because some compounds interfere with assays. For example, the sulfhydryl-scavenging assay used to detect histone acetyltransferase inhibition can give a false positive result if the test compound reacts covalently with cysteines.[42] BiopiracyLook up biopiracy in Wiktionary, the free dictionary. The term biopiracy was coined by Pat Mooney,[43] to describe a practice in which indigenous knowledge of nature, originating with indigenous peoples, is used by others for profit, without authorization or compensation to the indigenous people themselves.[44] For example, when bioprospectors draw on indigenous knowledge of medicinal plants which is later patented by medical companies without recognizing the fact that the knowledge is not new or invented by the patenter, this deprives the indigenous community of their potential rights to the commercial product derived from the technology that they themselves had developed.[45] Critics of this practice, such as Greenpeace,[46] claim these practices contribute to inequality between developing countries rich in biodiversity, and developed countries hosting biotech firms.[45] In the 1990s many large pharmaceutical and drug discovery companies responded to charges of biopiracy by ceasing work on natural products, turning to combinatorial chemistry to develop novel compounds.[43] Famous cases of biopiracy The rosy periwinkleThe rosy periwinkle case dates from the 1950s. The rosy periwinkle, while native to Madagascar, had been widely introduced into other tropical countries around the world well before the discovery of vincristine. Different countries are reported as having acquired different beliefs about the medical properties of the plant.[47] This meant that researchers could obtain local knowledge from one country and plant samples from another. The use of the plant for diabetes was the original stimulus for research. Effectiveness in the treatment of both Hodgkin lymphoma and leukemia were discovered instead.[48] The Hodgkin lymphoma chemotherapeutic drug vinblastine is derivable from the rosy periwinkle.[49] The Maya ICBG controversyThe Maya ICBG bioprospecting controversy took place in 1999–2000, when the International Cooperative Biodiversity Group led by ethnobiologist Brent Berlin was accused of being engaged in unethical forms of bioprospecting by several NGOs and indigenous organizations. The ICBG aimed to document the biodiversity of Chiapas, Mexico, and the ethnobotanical knowledge of the indigenous Maya people – in order to ascertain whether there were possibilities of developing medical products based on any of the plants used by the indigenous groups.[50][51] The Maya ICBG case was among the first to draw attention to the problems of distinguishing between benign forms of bioprospecting and unethical biopiracy, and to the difficulties of securing community participation and prior informed consent for would-be bioprospectors.[52] The neem tree In 1994, the U.S. Department of Agriculture and W. R. Grace and Company received a European patent on methods of controlling fungal infections in plants using a composition that included extracts from the neem tree (Azadirachta indica), which grows throughout India and Nepal.[53][54][55] In 2000 the patent was successfully opposed by several groups from the EU and India including the EU Green Party, Vandana Shiva, and the International Federation of Organic Agriculture Movements (IFOAM) on the basis that the fungicidal activity of neem extract had long been known in Indian traditional medicine.[55] WR Grace appealed and lost in 2005.[56] Basmati riceIn 1997, the US corporation RiceTec (a subsidiary of RiceTec AG of Liechtenstein) attempted to patent certain hybrids of basmati rice and semidwarf long-grain rice.[57] The Indian government challenged this patent and, in 2002, fifteen of the patent's twenty claims were invalidated.[58] The Enola bean The Enola bean is a variety of Mexican yellow bean, so called after the wife of the man who patented it in 1999.[59] The allegedly distinguishing feature of the variety is seeds of a specific shade of yellow. The patent-holder subsequently sued a large number of importers of Mexican yellow beans with the following result: "...export sales immediately dropped over 90% among importers that had been selling these beans for years, causing economic damage to more than 22,000 farmers in northern Mexico who depended on sales of this bean."[60] A lawsuit was filed on behalf of the farmers and, in 2005, the US-PTO ruled in favor of the farmers. In 2008, the patent was revoked.[61] Hoodia gordonii Hoodia gordonii, a succulent plant, originates from the Kalahari Desert of South Africa. For generations it has been known to the traditionally living San people as an appetite suppressant. In 1996 South Africa's Council for Scientific and Industrial Research began working with companies, including Unilever, to develop dietary supplements based on Hoodia.[62][63][64][65] Originally the San people were not scheduled to receive any benefits from the commercialization of their traditional knowledge, but in 2003 the South African San Council made an agreement with CSIR in which they would receive from 6 to 8% of the revenue from the sale of Hoodia products.[66] In 2008 after having invested €20 million in R&D on Hoodia as a potential ingredient in dietary supplements for weight loss, Unilever terminated the project because their clinical studies did not show that Hoodia was safe and effective enough to bring to market.[67] Further casesThe following is a selection of further recent cases of biopiracy. Most of them do not relate to traditional medicines.
Legal and political aspects
Patent lawOne common misunderstanding is that pharmaceutical companies patent the plants they collect. While obtaining a patent on a naturally occurring organism as previously known or used is not possible, patents may be taken out on specific chemicals isolated or developed from plants. Often these patents are obtained with a stated and researched use of those chemicals.[citation needed] Generally the existence, structure and synthesis of those compounds is not a part of the indigenous medical knowledge that led researchers to analyze the plant in the first place. As a result, even if the indigenous medical knowledge is taken as prior art, that knowledge does not by itself make the active chemical compound "obvious," which is the standard applied under patent law. In the United States, patent law can be used to protect "isolated and purified" compounds – even, in one instance, a new chemical element (see USP 3,156,523). In 1873, Louis Pasteur patented a "yeast" which was "free from disease" (patent #141072). Patents covering biological inventions have been treated similarly. In the 1980 case of Diamond v. Chakrabarty, the Supreme Court upheld a patent on a bacterium that had been genetically modified to consume petroleum, reasoning that U.S. law permits patents on "anything under the sun that is made by man." The United States Patent and Trademark Office (USPTO) has observed that "a patent on a gene covers the isolated and purified gene but does not cover the gene as it occurs in nature".[76] Also possible under US law is patenting a cultivar, a new variety of an existing organism. The patent on the Enola bean (now revoked)[77] was an example of this sort of patent. The intellectual property laws of the US also recognize plant breeders' rights under the Plant Variety Protection Act, 7 U.S.C. §§ 2321–2582.[78] Convention on Biological Diversity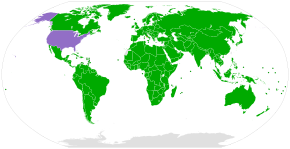 Parties to the CBD[79] Signed, but not ratified[79] Non-signatory[79] The Convention on Biological Diversity (CBD) came into force in 1993. It secured rights to control access to genetic resources for the countries in which those resources are located. One objective of the CBD is to enable lesser-developed countries to better benefit from their resources and traditional knowledge. Under the rules of the CBD, bioprospectors are required to obtain informed consent to access such resources, and must share any benefits with the biodiversity-rich country.[80] However, some critics believe that the CBD has failed to establish appropriate regulations to prevent biopiracy.[81] Others claim that the main problem is the failure of national governments to pass appropriate laws implementing the provisions of the CBD.[82] The Nagoya Protocol to the CBD, which came into force in 2014, provides further regulations.[83] The CBD has been ratified, acceded or accepted by 196 countries and jurisdictions globally, with exceptions including the Holy See and United States.[79] Bioprospecting contractsThe requirements for bioprospecting as set by CBD has created a new branch of international patent and trade law, bioprospecting contracts.[2] Bioprospecting contracts lay down the rules of benefit sharing between researchers and countries, and can bring royalties to lesser-developed countries. However, although these contracts are based on prior informed consent and compensation (unlike biopiracy), every owner or carrier of an indigenous knowledge and resources are not always consulted or compensated,[84] as it would be difficult to ensure every individual is included.[85] Because of this, some have proposed that the indigenous or other communities form a type of representative micro-government that would negotiate with researchers to form contracts in such a way that the community benefits from the arrangements.[85] Unethical bioprospecting contracts (as distinct from ethical ones) can be viewed as a new form of biopiracy.[81] An example of a bioprospecting contract is the agreement between Merck and INBio of Costa Rica.[86] Traditional knowledge databaseDue to previous cases of biopiracy and to prevent further cases, the Government of India has converted traditional Indian medicinal information from ancient manuscripts and other resources into an electronic resource; this resulted in the Traditional Knowledge Digital Library in 2001.[87] The texts are being recorded from Tamil, Sanskrit, Urdu, Persian and Arabic; made available to patent offices in English, German, French, Japanese and Spanish. The aim is to protect India's heritage from being exploited by foreign companies.[88] Hundreds of yoga poses are also kept in the collection.[88] The library has also signed agreements with leading international patent offices such as European Patent Office (EPO), United Kingdom Trademark & Patent Office (UKTPO) and the United States Patent and Trademark Office to protect traditional knowledge from biopiracy as it allows patent examiners at International Patent Offices to access TKDL databases for patent search and examination purposes.[73][89][90] See alsoLook up bioprospecting in Wiktionary, the free dictionary.
References
Bibliography and resources
External links
|


