|
List of measuring instruments
 A measuring instrument is a device to measure a physical quantity. In the physical sciences, quality assurance, and engineering, measurement is the activity of obtaining and comparing physical quantities of real-world objects and events. Established standard objects and events are used as units, and the process of measurement gives a number relating the item under study and the referenced unit of measurement. Measuring instruments, and formal test methods which define the instrument's use, are the means by which these relations of numbers are obtained. All measuring instruments are subject to varying degrees of instrument error and measurement uncertainty. These instruments may range from simple objects such as rulers and stopwatches to electron microscopes and particle accelerators. Virtual instrumentation is widely used in the development of modern measuring instruments. Time In the past, a common time measuring instrument was the sundial. Today, the usual measuring instruments for time are clocks and watches. For highly accurate measurement of time an atomic clock is used. Stopwatches are also used to measure time in some sports. Energy  Energy is measured by an energy meter. Examples of energy meters include: Electricity meterAn electricity meter measures energy directly in kilowatt-hours. Gas meterA gas meter measures energy indirectly by recording the volume of gas used. This figure can then be converted to a measure of energy by multiplying it by the calorific value of the gas. Power (flux of energy)A physical system that exchanges energy may be described by the amount of energy exchanged per time-interval, also called power or flux of energy.
For the ranges of power-values see: Orders of magnitude (power). ActionAction describes energy summed up over the time a process lasts (time integral over energy). Its dimension is the same as that of an angular momentum.
GeometryDimensions (size)Length (distance)For the ranges of length-values see: Orders of magnitude (length) AreaFor the ranges of area-values see: Orders of magnitude (area) Volume
If the mass density of a solid is known, weighing allows to calculate the volume. For the ranges of volume-values see: Orders of magnitude (volume) Angle
Orientation in three-dimensional spaceSee also the section about navigation below. LevelDirectionCoordinatesMechanicsThis includes basic quantities found in classical- and continuum mechanics; but strives to exclude temperature-related questions or quantities. Mass- or volume flow measurementSpeed or velocity (flux of length)
For the ranges of speed-values see: Orders of magnitude (speed) AccelerationMass
For the ranges of mass-values see: Orders of magnitude (mass) Linear momentumForce (flux of linear momentum) Pressure (flux density of linear momentum)
For the ranges of pressure-values see: Orders of magnitude (pressure) Angular velocity or rotations per time unitFor the value-ranges of angular velocity see: Orders of magnitude (angular velocity) For the ranges of frequency see: Orders of magnitude (frequency) TorqueEnergy carried by mechanical quantities, mechanical work
Electricity, electronics, and electrical engineering Considerations related to electric charge dominate electricity and electronics. Electrical charges interact via a field. That field is called electric field.If the charge doesn't move. If the charge moves, thus realizing an electric current, especially in an electrically neutral conductor, that field is called magnetic. Electricity can be given a quality — a potential. And electricity has a substance-like property, the electric charge. Energy (or power) in elementary electrodynamics is calculated by multiplying the potential by the amount of charge (or current) found at that potential: potential times charge (or current). (See Classical electromagnetism and Covariant formulation of classical electromagnetism)  Electric charge
For the ranges of charge values see: Orders of magnitude (charge) Electric current (current of charge)Voltage (electric potential difference)
Energy carried by electricity or electric energyElectric field (negative gradient of electric potential, voltage per length)See also the relevant section in the article about the magnetic field. For the ranges of magnetic field see: Orders of magnitude (magnetic field) Combination instruments
Temperature-related considerations dominate thermodynamics. There are two distinct thermal properties: A thermal potential — the temperature. For example: A glowing coal has a different thermal quality than a non-glowing one. And a substance-like property, — the entropy; for example: One glowing coal won't heat a pot of water, but a hundred will. Energy in thermodynamics is calculated by multiplying the thermal potential by the amount of entropy found at that potential: temperature times entropy. Entropy can be created by friction but not annihilated.
Imaging technology
See also Temperature measurement and Category:Thermometers. More technically related may be seen thermal analysis methods in materials science. For the ranges of temperature-values see: Orders of magnitude (temperature) Energy carried by entropy or thermal energy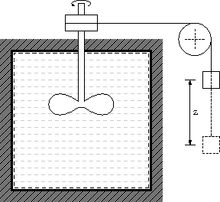 This includes thermal mass or temperature coefficient of energy, reaction energy, heat flow, ... Calorimeters are called passive if gauged to measure emerging energy carried by entropy, for example from chemical reactions. Calorimeters are called active or heated if they heat the sample, or reformulated: if they are gauged to fill the sample with a defined amount of entropy.
EntropyEntropy is accessible indirectly by measurement of energy and temperature. Entropy transferPhase change calorimeter's energy value divided by absolute temperature give the entropy exchanged. Phase changes produce no entropy and therefore offer themselves as an entropy measurement concept. Thus entropy values occur indirectly by processing energy measurements at defined temperatures, without producing entropy.
Entropy contentThe given sample is cooled down to (almost) absolute zero (for example by submerging the sample in liquid helium). At absolute zero temperature any sample is assumed to contain no entropy (see Third law of thermodynamics for further information). Then the following two active calorimeter types can be used to fill the sample with entropy until the desired temperature has been reached: (see also Thermodynamic databases for pure substances)
Entropy productionProcesses transferring energy from a non-thermal carrier to heat as a carrier do produce entropy (Example: mechanical/electrical friction, established by Count Rumford). Either the produced entropy or heat are measured (calorimetry) or the transferred energy of the non-thermal carrier may be measured.
Entropy lowering its temperature—without losing energy—produces entropy (Example: Heat conduction in an isolated rod; "thermal friction").
Concerning a given sample, a proportionality factor relating temperature change and energy carried by heat. If the sample is a gas, then this coefficient depends significantly on being measured at constant volume or at constant pressure. (The terminology preference in the heading indicates that the classical use of heat bars it from having substance-like properties.)
Specific temperature coefficient of energy or "specific heat capacity"The temperature coefficient of energy divided by a substance-like quantity (amount of substance, mass, volume) describing the sample. Usually calculated from measurements by a division or could be measured directly using a unit amount of that sample. For the ranges of specific heat capacities see: Orders of magnitude (specific heat capacity) Melting temperature (of a solid)
Boiling temperature (of a liquid)
See also Thermal analysis, Heat. More on continuum mechanicsThis includes mostly instruments which measure macroscopic properties of matter: In the fields of solid-state physics; in condensed matter physics which considers solids, liquids, and in-betweens exhibiting for example viscoelastic behavior; and furthermore, in fluid mechanics, where liquids, gases, plasmas, and in-betweens like supercritical fluids are studied. This refers to particle density of fluids and compact(ed) solids like crystals, in contrast to bulk density of grainy or porous solids.
For the ranges of density-values see: Orders of magnitude (density) Hardness of a solidShape and surface of a solid
Deformation of condensed matter
Elasticity of a solid (elastic moduli)
Plasticity of a solid Tensile strength, ductility, or malleability of a solidGranularity of a solid or of a suspensionViscosity of a fluidSurface tension of liquidsImaging technology
This section and the following sections include instruments from the wide field of Category:Materials science, materials science. More on electric properties of condensed matter, gas Such measurements also allow to access values of molecular dipoles. For other methods see the section in the article about magnetic susceptibility. See also Category:Electric and magnetic fields in matter Substance potential or chemical potential or molar Gibbs energyPhase conversions like changes of aggregate state, chemical reactions or nuclear reactions transmuting substances, from reactants into products, or diffusion through membranes have an overall energy balance. Especially at constant pressure and constant temperature, molar energy balances define the notion of a substance potential or chemical potential or molar Gibbs energy, which gives the energetic information about whether the process is possible or not - in a closed system. Energy balances that include entropy consist of two parts: A balance that accounts for the changed entropy content of the substances, and another one that accounts for the energy freed or taken by that reaction itself, the Gibbs energy change. The sum of reaction energy and energy associated to the change of entropy content is also called enthalpy. Often the whole enthalpy is carried by entropy and thus measurable calorimetrically. For standard conditions in chemical reactions either molar entropy content and molar Gibbs energy with respect to some chosen zero point are tabulated. Or molar entropy content and molar enthalpy with respect to some chosen zero are tabulated. (See Standard enthalpy change of formation and Standard molar entropy) The substance potential of a redox reaction is usually determined electrochemically current-free using reversible cells. Other values may be determined indirectly by calorimetry. Also by analyzing phase-diagrams. Sub-microstructural properties of condensed matter, gas
Imaging technology, microscope
(See also Spectroscopy and List of materials analysis methods.) Sound, compression waves in matterMicrophones in general, sometimes their sensitivity is increased by the reflection- and concentration principle realized in acoustic mirrors.
  Light and radiation without a rest mass, non-ionizing
(for lux meter, see the section about human senses and human body) See also Category:Optical devices Pressure (current density of linear momentum)The measure of the total power of light emitted.
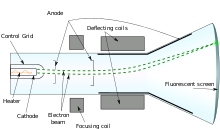 Radiation with a rest mass, particle radiation
 Ionizing radiation includes rays of "particles" as well as rays of "waves". Especially X-rays and gamma rays transfer enough energy in non-thermal, (single-) collision processes to separate electron(s) from an atom.  Particle and ray flux
Identification and contentThis could include chemical substances, rays of any kind, elementary particles, and quasiparticles. Many measurement devices outside this section may be used or at least become part of an identification process. For identification and content concerning chemical substances, see also Analytical chemistry, List of chemical analysis methods, and List of materials analysis methods.
pH: Concentration of protons in a solution
 Brightness: photometryPhotometry is the measurement of light in terms of its perceived brightness to the human eye. Photometric quantities derive from analogous radiometric quantities by weighting the contribution of each wavelength by a luminosity function that models the eye's spectral sensitivity. For the ranges of possible values, see the orders of magnitude in: illuminance, luminance, and luminous flux.
Color: colorimetry
Radar brightness: radiometrySynthetic Aperture Radar (SAR) instruments measure radar brightness, Radar Cross Section (RCS), which is a function of the reflectivity and moisture of imaged objects at wavelengths which are too long to be perceived by the human eye. Black pixels mean no reflectivity (e.g. water surfaces), white pixels mean high reflectivity (e.g. urban areas). Colored pixels can be obtained by combining three gray-scaled images which usually interpret the polarization of electromagnetic waves. The combination R-G-B = HH-HV-VV combines radar images of waves sent and received horizontally (HH), sent horizontally and received vertically (HV) and sent and received vertically (VV). The calibration of such instruments is done by imaging objects (calibration targets) whose radar brightness is known. Hearing
Smell
Temperature (sense and body)
Circulatory system (mainly heart and blood vessels for distributing substances fast)Blood-related parameters are listed in a blood test.
Respiratory system (lung and airways controlling the breathing process) Concentration or partial pressure of carbon dioxide in the respiratory gasesNervous system (nerves transmitting and processing information electrically)
Musculoskeletal system (muscles and bones for movement)
See also: Category:Physiological instruments and Category:Medical testing equipment. See also Category:Meteorological instrumentation and equipment. Navigation and surveyingSee also Category:Navigational equipment and Category:Navigation. See also Surveying instruments. AstronomySee also Astronomical instruments and Category:Astronomical observatories. MilitarySome instruments, such as telescopes and sea navigation instruments, have had military applications for many centuries. However, the role of instruments in military affairs rose exponentially with the development of technology via applied science, which began in the mid-19th century and has continued through the present day. Military instruments as a class draw on most of the categories of instrument described throughout this article, such as navigation, astronomy, optics, and imaging, and the kinetics of moving objects. Common abstract themes that unite military instruments are seeing into the distance, seeing in the dark, knowing an object's geographic location, and knowing and controlling a moving object's path and destination. Special features of these instruments may include ease of use, speed, reliability, and accuracy. Uncategorized, specialized, or generalized application
Alphabetical listing
See also
NotesThe alternate spelling "-metre" is never used when referring to a measuring device. ReferencesExternal links
|
Portal di Ensiklopedia Dunia