|
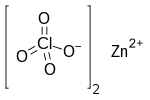
Zinc perchlorate
Zinc perchlorate is the inorganic compound with the chemical formula Zn(ClO4)2 which forms the hexahydrate.[1][2] SynthesisZinc perchlorate can be prepared by dissolving zinc oxide or zinc carbonate in perchloric acid:[3]
Chemical propertiesThe compound decomposes when heated to high temperatures and may explode if heated too strongly. Like most other perchlorates such as copper perchlorate and lead perchlorate, zinc perchlorate is prone to deliquescence. Zinc perchlorate can form complexes with ligands such as 8-aminoquinoline, tricarbohydrazide, and tetraphenylethylene tetratriazole.[4] Physical propertiesThe compound forms a hexahydrate Zn(ClO Zinc perchlorate forms a hygroscopic colorless solid, odorless, soluble in water and low-weight alcohols. UsesZinc perchlorate is used as an oxidizing agent and catalyst. References
External links |
||||||||||||||||||||||||||||||||||||||||||||||||
Portal di Ensiklopedia Dunia