|
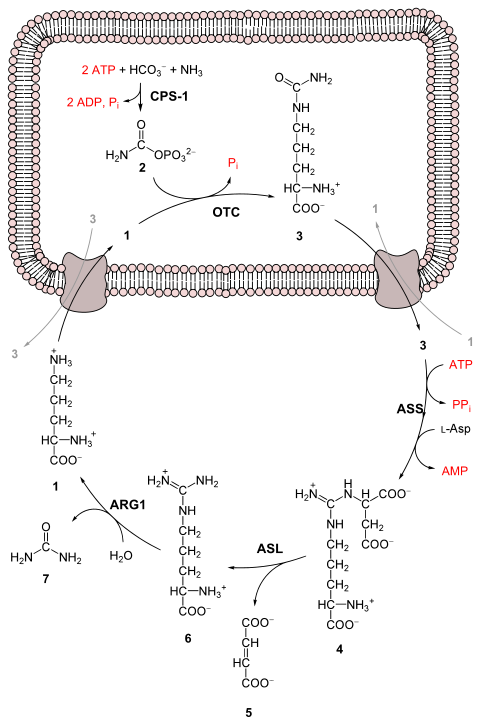
Urea cycleThe urea cycle (also known as the ornithine cycle) is a cycle of biochemical reactions that produces urea (NH2)2CO from ammonia (NH3). Animals that use this cycle, mainly amphibians and mammals, are called ureotelic. The urea cycle converts highly toxic ammonia to urea for excretion.[1] This cycle was the first metabolic cycle to be discovered by Hans Krebs and Kurt Henseleit in 1932,[2][3][4] five years before the discovery of the TCA cycle. The urea cycle was described in more detail later on by Ratner and Cohen. The urea cycle takes place primarily in the liver and, to a lesser extent, in the kidneys. FunctionAmino acid catabolism results in waste ammonia. All animals need a way to excrete this product. Most aquatic organisms, or ammonotelic organisms, excrete ammonia without converting it.[1] Organisms that cannot easily and safely remove nitrogen as ammonia convert it to a less toxic substance, such as urea, via the urea cycle, which occurs mainly in the liver. Urea produced by the liver is then released into the bloodstream, where it travels to the kidneys and is ultimately excreted in urine. The urea cycle is essential to these organisms, because if the nitrogen or ammonia is not eliminated from the organism it can be very detrimental.[5] In species including birds and most insects, the ammonia is converted into uric acid or its urate salt, which is excreted in solid form. Further, the urea cycle consumes acidic waste carbon dioxide by combining it with the basic ammonia, helping to maintain a neutral pH. ReactionsThe entire process converts two amino groups, one from NH+
First reaction: entering the urea cycleBefore the urea cycle begins ammonia is converted to carbamoyl phosphate. The reaction is catalyzed by carbamoyl phosphate synthetase I and requires the use of two ATP molecules.[1] The carbamoyl phosphate then enters the urea cycle. Steps of the urea cycle
Overall reaction equationIn the first reaction, NH+ Thus, the overall equation of the urea cycle is: Since fumarate is obtained by removing NH3 from aspartate (by means of reactions 3 and 4), and PPi + H2O → 2 Pi, the equation can be simplified as follows: Note that reactions related to the urea cycle also cause the production of 2 NADH, so the overall reaction releases slightly more energy than it consumes. The NADH is produced in two ways:
We can summarize this by combining the reactions:
The two NADH produced can provide energy for the formation of 5 ATP (cytosolic NADH provides 2.5 ATP with the malate-aspartate shuttle in human liver cell), a net production of two high-energy phosphate bond for the urea cycle. However, if gluconeogenesis is underway in the cytosol, the latter reducing equivalent is used to drive the reversal of the GAPDH step instead of generating ATP. The fate of oxaloacetate is either to produce aspartate via transamination or to be converted to phosphoenolpyruvate, which is a substrate for gluconeogenesis. Products of the urea cycleAs stated above many vertebrates use the urea cycle to create urea out of ammonium so that the ammonium does not damage the body. Though this is helpful, there are other effects of the urea cycle. For example: consumption of two ATP, production of urea, generation of H+, the combining of HCO−3 and NH+4 to forms where it can be regenerated, and finally the consumption of NH+4.[9] RegulationN-Acetylglutamic acidThe synthesis of carbamoyl phosphate and the urea cycle are dependent on the presence of N-acetylglutamic acid (NAcGlu), which allosterically activates CPS1. NAcGlu is an obligate activator of carbamoyl phosphate synthetase.[10] Synthesis of NAcGlu by N-acetylglutamate synthase (NAGS) is stimulated by both Arg, allosteric stimulator of NAGS, and Glu, a product in the transamination reactions and one of NAGS's substrates, both of which are elevated when free amino acids are elevated. So Glu not only is a substrate for NAGS but also serves as an activator for the urea cycle. Substrate concentrationsThe remaining enzymes of the cycle are controlled by the concentrations of their substrates. Thus, inherited deficiencies in cycle enzymes other than ARG1 do not result in significant decreases in urea production (if any cycle enzyme is entirely missing, death occurs shortly after birth). Rather, the deficient enzyme's substrate builds up, increasing the rate of the deficient reaction to normal. The anomalous substrate buildup is not without cost, however. The substrate concentrations become elevated all the way back up the cycle to NH+ Although the root cause of NH+ Link with the citric acid cycleThe urea cycle and the citric acid cycle are independent cycles but are linked. One of the nitrogen atoms in the urea cycle is obtained from the transamination of oxaloacetate to aspartate.[11] The fumarate that is produced in step three is also an intermediate in the citric acid cycle and is returned to that cycle.[11] Urea cycle disordersUrea cycle disorders are rare and affect about one in 35,000 people in the United States.[12] Genetic defects in the enzymes involved in the cycle can occur, which usually manifest within a few days after birth.[5] The recently born child will typically experience varying bouts of vomiting and periods of lethargy.[5] Ultimately, the infant may go into a coma and develop brain damage.[5] New-borns with UCD are at a much higher risk of complications or death due to untimely screening tests and misdiagnosed cases. The most common misdiagnosis is neonatal sepsis. Signs of UCD can be present within the first 2 to 3 days of life, but the present method to get confirmation by test results can take too long.[13] This can potentially cause complications such as coma or death.[13] Urea cycle disorders may also be diagnosed in adults, and symptoms may include delirium episodes, lethargy, and symptoms similar to that of a stroke.[14] On top of these symptoms, if the urea cycle begins to malfunction in the liver, the patient may develop cirrhosis.[15] This can also lead to sarcopenia (the loss of muscle mass).[15] Mutations lead to deficiencies of the various enzymes and transporters involved in the urea cycle, and cause urea cycle disorders.[1] If individuals with a defect in any of the six enzymes used in the cycle ingest amino acids beyond what is necessary for the minimum daily requirements, then the ammonia that is produced will not be able to be converted to urea. These individuals can experience hyperammonemia, or the build-up of a cycle intermediate. Individual disorders
All urea cycle defects, except OTC deficiency, are inherited in an autosomal recessive manner. OTC deficiency is inherited as an X-linked recessive disorder, although some females can show symptoms. Most urea cycle disorders are associated with hyperammonemia, however argininemia and some forms of argininosuccinic aciduria do not present with elevated ammonia. Additional images
References
External links
|
Portal di Ensiklopedia Dunia