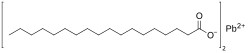
Lead stearate
Names
Other names
Lead(2+) octadecanoate, lead(II) stearate, lead distearate
Identifiers
ChemSpider
ECHA InfoCard 100.012.733
EC Number
UNII
InChI=1S/2C18H36O2.Pb/c2*1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20;/h2*2-17H2,1H3,(H,19,20);/q;;+2/p-2
Key: UQLDLKMNUJERMK-UHFFFAOYSA-L
CCCCCCCCCCCCCCCCCC(=O)[O-].CCCCCCCCCCCCCCCCCC(=O)[O-].[Pb+2]
Properties
C36 70 4
Molar mass
774.14
Appearance
White powder
Density
1.4 g/cm3
Melting point
115.7 °C (240.3 °F; 388.8 K)
Boiling point
359.4 °C (678.9 °F; 632.5 K)
Slightly soluble
Hazards
GHS labelling
Danger
H302 , H332 , H360 , H373
P260 , P261 , P281 , P304 , P340 , P405 , P501
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Lead stearate is a metal-organic compound , a salt of lead and stearic acid with the chemical formula C36 70 4 .[ 1] metallic soap , i.e. a metal derivative of a fatty acid .[ 2]
Synthesis
The compound can be prepared by reacting stearic acid , lead(II) oxide , and a catalyst acetic acid .[ 3]
2
C
17
H
35
C
O
O
H
+
P
b
O
⟶
(
C
17
H
35
C
O
O
)
2
P
b
+
H
2
O
{\displaystyle \mathrm {2\ C_{17}H_{35}COOH+PbO\longrightarrow (C_{17}H_{35}COO)_{2}Pb+\ H_{2}O} }
Also, an exchange reaction between lead(II) acetate and sodium stearate :
P
b
(
C
H
3
C
O
O
)
2
+
2
N
a
C
18
H
35
O
2
→
P
b
(
C
18
H
35
O
2
)
2
↓
+
2
C
H
3
C
O
O
N
a
{\displaystyle {\mathsf {Pb(CH_{3}COO)_{2}+2NaC_{18}H_{35}O_{2}\ {\xrightarrow {}}\ Pb(C_{18}H_{35}O_{2})_{2}\downarrow +2CH_{3}COONa}}}
Physical properties
White powder with a slight fatty odor. Sinks in water.[ 4]
Slightly soluble in water.[ 1] ethanol .
Uses
The compound is used as a drier in oil paints and varnishes to speed the polymerization and oxidation processes. Also used as a lubricant and stabilizer in vinyl polymers and as a corrosion inhibitor in petroleum products.[ 5] [ 6] [ 7]
References
Salts and covalent derivatives of the
stearate ion