Cobalt(II,III) oxide[ 1]
Cobalt(II,III) oxide
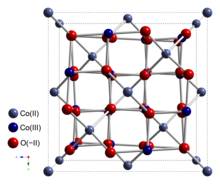
Ball-and-stick model of the unit cell of Co3O4
Names
IUPAC name
cobalt(II) dicobalt(III) oxide
Other names
cobalt oxide, cobalt(II,III) oxide, cobaltosic oxide, tricobalt tetroxide
Identifiers
ChemSpider
ECHA InfoCard 100.013.780
EC Number
RTECS number
UNII
InChI=1S/3Co.4O
Y Key: LBFUKZWYPLNNJC-UHFFFAOYSA-N
Y InChI=1/3Co.4O/rCo2O3.CoO/c3-1-5-2-4;1-2
Key: LBFUKZWYPLNNJC-PMPQCLQHAA
Properties
Co3 O4 CoO.Co2 O3
Molar mass
240.80 g/mol
Appearance
black solid
Density
6.07 g/cm3 [ 2]
Melting point
895 °C (1,643 °F; 1,168 K)
Boiling point
900 °C (1,650 °F; 1,170 K) (decomposes)
Insoluble
Solubility
soluble (with degradation) in acids and alkalis
+7380·10−6 cm3 /mol
Structure
cubic
Fd3 m, No. 227[ 3]
Hazards
GHS labelling
Danger
H317 , H334 , H350 , H411
P261 , P273 , P284 , P304+P340 , P342+P311
NFPA 704
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Cobalt(II,III) oxide is an inorganic compound with the formula Co3 O4 . It is one of two well characterized cobalt oxides . It is a black antiferromagnetic solid. As a mixed valence compound , its formula is sometimes written as CoII CoIII 2 O4 and sometimes as CoO•Co2 O3 .[ 4]
Structure
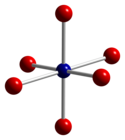
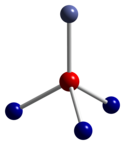
Co3 O4 adopts the normal spinel structure , with Co2+ ions in tetrahedral interstices and Co3+ ions in the octahedral interstices of the cubic close-packed lattice of oxide anions.[ 4]
Synthesis
Cobalt(II) oxide , CoO, converts to Co3 O4 upon heating at around 600–700 °C in air.[ 4] [ 4] [ 5]
2 Co3 O4 ⇌ 6 CoO + O2
Applications
Cobalt(II,III) oxide is used as a blue coloring agent for pottery enamel and glass , as an alternative to cobalt(II) oxide.[ 6]
Cobalt(II,III) oxide is used as an electrode in some lithium-ion batteries , possibly in the form of cobalt oxide nanoparticles .
Safety
Cobalt compounds are potentially poisonous in large amounts.[ 7]
See also
References
^ "Cobalt(II,III) oxide 203114" . Sigma-Aldrich .^ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics CRC Press . ISBN 0-8493-0487-3 ^ "mp-18748: Co3O4 (cubic, Fd-3m, 227)" . materialsproject.org . Retrieved 2019-12-20 .^ a b c d Greenwood, Norman N. ; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann . p. 1118. ISBN 978-0-08-037941-8 ^ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. p. 1520.
^ Frank Hamer, Janet Hamer (2004): The Potter's Dictionary of Materials and Techniques ISBN 0812238109
^ MSDS [permanent dead link ]
Cobalt(I) Cobalt(II) Cobalt(0,III) Cobalt(II,III) Cobalt(III) Cobalt(III,IV) Cobalt(IV) Cobalt(V)
Mixed oxidation states +1 oxidation state +2 oxidation state +3 oxidation state +4 oxidation state +5 oxidation state +6 oxidation state +7 oxidation state +8 oxidation state Related