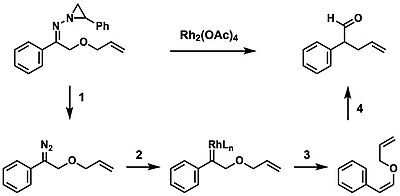
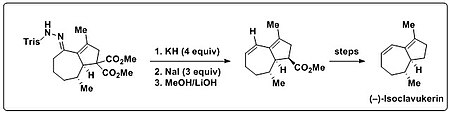
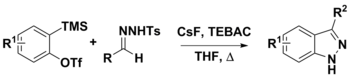Bamford–Stevens reaction
| |||||||||||||||
Read other articles:

恩维尔·霍查Enver Hoxha霍查官方肖像照(摄于1980年代初)阿尔巴尼亚共产党中央委员会总书记任期1943年3月—1948年11月[1]前任無(首任)继任本人(劳动党中央委员会总书记)阿尔巴尼亚劳动党中央委员会总书记任期1948年11月—1954年7月[1]前任本人(共产党中央委员会总书记)继任本人(劳动党中央委员会第一书记)阿尔巴尼亚劳动党中央委员会第一书记任期1954年7�…

Vous lisez un « bon article » labellisé en 2007. Pour les articles homonymes, voir Juste. Juste parmi les nations Diplôme de Yad Vashem pour Auguste et Jeanne Bieber. Conditions Décerné par Israël Type Diplôme et médaille Éligibilité Ne pas être juif et avoir apporté une aide, dans des situations où les Juifs étaient menacés de mort, au risque de sa propre vie et de celle de ses proches. Statistiques Création 1963 Total 28 217 Inférieur Équivalent Supérieur mod…

Questa voce sull'argomento cestisti statunitensi è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. Segui i suggerimenti del progetto di riferimento. Kris Clyburn Nazionalità Stati Uniti Altezza 198 cm Peso 82 kg Pallacanestro Ruolo Guardia Squadra Mitteldeutscher BC CarrieraGiovanili Romulus Senior High School2015-2016Ranger College2016-2019 UNLV R. RebelsSquadre di club 2019-2020 Astoria Bydgoszcz21 (322)2020-2021 Cmoki Minsk202…

Questa voce o sezione sull'argomento storia è priva o carente di note e riferimenti bibliografici puntuali. Sebbene vi siano una bibliografia e/o dei collegamenti esterni, manca la contestualizzazione delle fonti con note a piè di pagina o altri riferimenti precisi che indichino puntualmente la provenienza delle informazioni. Puoi migliorare questa voce citando le fonti più precisamente. Segui i suggerimenti del progetto di riferimento. Mappa della Grande Serbia delimitata dalla linea Vi…

This article is about intercity bus travel. For Chinese-owned intracity public transit, see Dollar vans in the New York metropolitan area § Chinatown vans. Passengers waiting to board a Travel Pack bus on Mulberry Street in Manhattan en route to Boston in 2004 Passengers waiting at the now-defunct Fung Wah Bus Transportation ticket window on Canal Street at the Bowery in Manhattan's Chinatown Eastern Bus MCI 102DL3 coach boarding passengers in Manhattan's Chinatown 2010 schematic map of fo…

العلاقات الدومينيكانية السريلانكية جمهورية الدومينيكان سريلانكا جمهورية الدومينيكان سريلانكا تعديل مصدري - تعديل العلاقات الدومينيكانية السريلانكية هي العلاقات الثنائية التي تجمع بين جمهورية الدومينيكان وسريلانكا.[1][2][3][4][5] مقارنة …

Football rivalry between the national football teams of England and Scotland England–Scotland football rivalryNewspaper advertisement for the first official international football match.LocationEurope (UEFA)Teams England ScotlandFirst meeting30 November 1872(SCO 0–0 ENG)Latest meeting12 September 2023(SCO 1–3 ENG)StatisticsMeetings total116Most winsEngland (49)All-time series (none-international fixture only)49–41–26 (England)Largest victoryENG 9–3 SCO(15 April 1961)Eng…

Ini adalah nama Maluku, Ambon marganya adalah Rumasukun Ridwan Rumasukun Penjabat Gubernur PapuaPetahanaMulai menjabat 5 September 2023(Pelaksana Harian: 11 Januari – 5 September 2023)PresidenJoko WidodoPendahuluLukas EnembePenggantiPetahanaSekretaris Daerah PapuaMasa jabatan14 Juli 2021 – 12 Januari 2023PresidenJoko WidodoGubernurLukas EnembePendahuluDance Yulian FlassyPenggantiDerek Hegemur (Plh.)Masa jabatan7 April 2020 – 25 September 2020PendahuluHery DosinaenPeng…

British economist and politician (1772–1823) For other people named David Ricardo, see David Ricardo (disambiguation). The Right HonourableDavid RicardoPortrait by Thomas Phillips, c. 1821Member of Parliament for PortarlingtonIn office20 February 1819 – 11 September 1823Preceded byRichard SharpSucceeded byJames Farquhar Personal detailsBorn(1772-04-18)18 April 1772London, EnglandDied11 September 1823(1823-09-11) (aged 51)Gatcombe Park, Gloucestershire, EnglandPolitical partyWhi…

أغوستين كانوبيو معلومات شخصية الميلاد 1 أكتوبر 1998 (26 سنة)[1] مونتفيدو[2] الطول 1.75 م (5 قدم 9 بوصة) مركز اللعب مهاجم الجنسية الأوروغواي الأب أوزفالدو كانوبيو معلومات النادي النادي الحالي أتلتيكو باراناينسي الرقم 9 مسيرة الشباب سنوات فريق 2011–2016 سنترو …

العلاقات الأرجنتينية التوفالية الأرجنتين توفالو الأرجنتين توفالو تعديل مصدري - تعديل العلاقات الأرجنتينية التوفالية هي العلاقات الثنائية التي تجمع بين الأرجنتين وتوفالو.[1][2][3][4][5] مقارنة بين البلدين هذه مقارنة عامة ومرجعية للدولتين: �…

U.S. guided-missile frigates under construction An artist's rendering of the final Constellation-class design Class overview NameConstellation class BuildersFincantieri Marinette Marine Operators United States Navy (projected) Preceded by Freedom and Independence classes Oliver Hazard Perry class (last class with FFG hull code) Cost US$1.28 billion for the first ship[1] US$1.05 billion for the second ship[1] Built2022–present In commission2029 (planned)[2]…

Petite fleur ist der Titel eines 1952 von Sidney Bechet komponierten und durch Chris Barber’s Jazz Band im Jahre 1959 bekannt gewordenen Jazzstandards. Inhaltsverzeichnis 1 Entstehungsgeschichte 2 Weitere Aufnahmen 3 Erfolg 4 Weblinks 5 Einzelnachweise Entstehungsgeschichte Der in New Orleans aufgewachsene ehemalige Straßenmusiker Sidney Bechet reiste im Mai 1949 zum Festival International 1949 de Jazz nach Frankreich. Als er auf unerwartet große Resonanz stieß, blieb er in Frankreich, wo e…

Book by Garth Risk Hallberg City on Fire First edition (US)AuthorGarth Risk HallbergLanguageEnglishGenreFictionPublisherAlfred A. Knopf (US)Jonathan Cape (UK)Publication date2015Publication placeUnited StatesMedia typePrint (Hardback & Paperback)Pages927ISBN978-0-385-35377-9 City on Fire is a 2015 novel by Garth Risk Hallberg, published by Alfred A. Knopf.[1][2][3] The novel takes place in New York City in the 1970s. It is Hallberg's first published novel.[1&…

يفتقر محتوى هذه المقالة إلى الاستشهاد بمصادر. فضلاً، ساهم في تطوير هذه المقالة من خلال إضافة مصادر موثوق بها. أي معلومات غير موثقة يمكن التشكيك بها وإزالتها. (مارس 2016) هذه المقالة يتيمة إذ تصل إليها مقالات أخرى قليلة جدًا. فضلًا، ساعد بإضافة وصلة إليها في مقالات متعلقة بها. (نو…

1976 Republican Party presidential primaries ← 1972 January 19 to June 8, 1976 1980 → 2,259 delegates to the Republican National Convention1,130 votes needed to win Candidate Gerald Ford Ronald Reagan Home state Michigan California Delegate count 1,121[1] 1,078[1] Contests won 27 24 Popular vote 5,529,899 4,760,222 Percentage 53.3% 45.9% First place by first-instance vote First place by delegate allocation First place by convention…

2018 Nollywood film directed by Omoni Oboli Moms at WarDirected byOmoni OboliWritten byNaz OnuzoProduced byMoses BabatopeStarringYul Edochie Eucharia Anunobi Funke AkindeleDistributed byFilm One DistributionRelease date 2018 (2018) Running time91 minutesCountryNigeriaLanguageEnglishBox officeNGN40,000,000 Moms at War is a 2018 Nigerian comedy drama movie directed by Omoni Oboli. It stars Funke Akindele as well as Michelle Dede, the former of whom won an award for her role as Best Actress in…

Questa voce sugli argomenti chimica teorica e chimica fisica è solo un abbozzo. Contribuisci a migliorarla secondo le convenzioni di Wikipedia. L'eptafluoruro di iodio, un composto ipervalente. Per molecola ipervalente si intende una molecola in cui uno dei suoi atomi presenta nel suo guscio elettronico più esterno più di otto elettroni.[1] Per tal motivo si dice anche che ha ottetto espanso. Composti con ottetto espanso non seguono la regola dell'ottetto, e sono ad esempio: TlI3…

2015 CampingWorld.com 500 at Talladega Race details[1][2][3][4][5][6][7][8][9] Race 32 of 36 in the 2015 NASCAR Sprint Cup Series Date October 25, 2015 (2015-10-25)Location Talladega Superspeedway in Lincoln, AlabamaCourse Permanent racing facility2.66 mi (4.28 km)Distance 196 laps, 521.36 mi (838.88 km)Scheduled Distance 188 laps, 500.08 mi (804.64 km)Average speed 167.311 mph (269.261 km/h)Pole pos…

Category 5 Atlantic hurricane in 2019 Not to be confused with Hurricane Lorena (2019). Hurricane Lorenzo Hurricane Lorenzo shortly before attaining Category 5 Strength on 29 SeptemberMeteorological historyFormed23 September 2019Extratropical2 October 2019Dissipated7 October 2019Category 5 major hurricane1-minute sustained (SSHWS/NWS)Highest winds160 mph (260 km/h)Lowest pressure925 mbar (hPa); 27.32 inHgOverall effectsFatalities19 direct, 1 indirect (20 total)D…