|
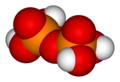
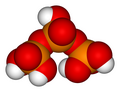
Phosphorus oxoacidIn chemistry, phosphorus oxoacid (or phosphorus acid) is a generic name for any acid whose molecule consists of atoms of phosphorus, oxygen, and hydrogen.[1] There is a potentially infinite number of such compounds. Some of them are unstable and have not been isolated, but the derived anions and organic groups are present in stable salts and esters. The most important ones—in biology, geology, industry, and chemical research—are the phosphoric acids, whose esters and salts are the phosphates. In general, any hydrogen atom bonded to an oxygen atom is acidic, meaning that the –OH group can lose a proton H+ ClassificationThe phosphorus oxoacids can be classified by the oxidation state(s) of the phosphorus atom(s), which may vary from +1 to +5. The oxygen atoms are usually in oxidation state -2, but may be in state -1 if the molecule includes peroxide groups. Oxidation state +1
Oxidation state +3
Oxidation state +4
Oxidation state +5The most important members of this group are the phosphoric acids, where each phosphorus atom bonded to four oxygen atoms, one of them through a double bond, arranged as the corners of a tetrahedron. Two or more of these PO These acids, and their esters and salts ("phosphates") include some of the best-known and most important compounds of phosphorus. 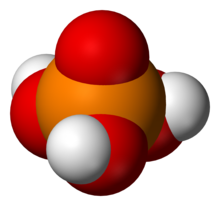 3PO 4. The simplest member of this class is:
The smallest compounds of this class with two or more phosphorus atoms are called "oligophosphoric acids", and the larger ones, with linear –P–O– backbones, are "polyphosphoric acids"; with no definite separation between the two. Some of the most important members are:
The backbone may be branched, as in:
The PO
Metaphosphoric acid is a general term for phosphoric acids with a single cycle, (–P(O)(OH)–O–)n, whose elemental formula is HPO
Another compound that may be included in this class is
Mixed oxidation statesSome phosphorus oxoacids have two or more P atoms in different oxidation states. One example is
 P4O10  See alsoReferences
Further reading
External links
|