18-甲氨基冠狗牙花定碱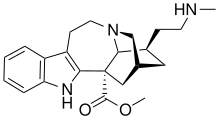 |
|
| CAS号 | 1807784-35-5  N[EPA] N[EPA] |
|---|
| PubChem CID | |
|---|
| ChemSpider | |
|---|
| CompTox Dashboard (EPA) | |
|---|
|
| 化学式 | C22H29N3O2 |
|---|
| 摩尔质量 | 367.49 g·mol−1 |
|---|
| 3D模型(JSmol) | |
|---|
c4cccc1c4[nH]c3c1CCN2CC(C5)CC3(C(=O)OC)C2C5CCNC
|
InChI=1S/C22H29N3O2/c1-23-9-7-15-11-14-12-22(21(26)27-2)19-17(8-10-25(13-14)20(15)22)16-5-3-4-6-18(16)24-19/h3-6,14-15,20,23-24H,7-13H2,1-2H3/t14-,15+,20+,22-/m1/s1  Y YKey:YKMOJVPLIUXEIP-SVBQBFEESA-N  Y Y
|
18-甲氨基冠狗牙花定碱(英語:18-Methylaminocoronaridine,缩写18-MAC)是一种有机化合物,分子式C22H29N3O2,属于伊波加因的衍生物,由佛蒙特大學的药理学家开发为药物。[1][2]
参考文献
- ^ Kuehne ME, He L, Jokiel PA, Pace CJ, Fleck MW, Maisonneuve IM, et al. Synthesis and biological evaluation of 18-methoxycoronaridine congeners. Potential antiaddiction agents. Journal of Medicinal Chemistry. June 2003, 46 (13): 2716–30. PMID 12801235. doi:10.1021/jm020562o.
- ^ Pace CJ, Glick SD, Maisonneuve IM, He LW, Jokiel PA, Kuehne ME, Fleck MW. Novel iboga alkaloid congeners block nicotinic receptors and reduce drug self-administration. European Journal of Pharmacology. May 2004, 492 (2–3): 159–67. PMID 15178360. doi:10.1016/j.ejphar.2004.03.062.