|
Vanadium(V) chloride
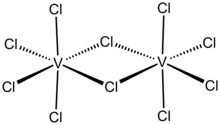
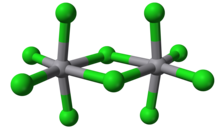
Vanadium(V) chloride is the inorganic compound with the formula VCl5. It is a black diamagnetic solid. The molecules adopt a bioctahedral structure similar to that of niobium(V) chloride.[1] Preparation and reactionsChlorine cannot oxidise vanadium(IV); chlorination of vanadium metal will yield only vanadium(IV) chloride. Vanadium(V) chloride is instead prepared from vanadium pentafluoride with excess boron trichloride as a chlorinating agent:
It is unstable at room temperature, releasing gaseous chlorine and giving vanadium(IV) chloride:
In contrast, the heavier analogues NbCl5 and TaCl5 are stable and not particularly oxidizing. References
|
||||||||||||||||||||||||||||||||