|
Naloxegol
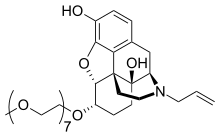
Naloxegol (INN; PEGylated naloxol;[4] trade names Movantik and Moventig) is a peripherally acting μ-opioid receptor antagonist developed by AstraZeneca, licensed from Nektar Therapeutics, for the treatment of opioid-induced constipation.[5] It was approved in 2014 in adult patients with chronic, non-cancer pain.[6] Doses of 25 mg were found safe and well tolerated for 52 weeks.[7] When given concomitantly with opioid analgesics, naloxegol reduced constipation-related side effects, while maintaining comparable levels of analgesia.[8] The most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting, and headache.[9] Naloxegol was previously a Schedule II drug in the United States because of its chemical similarity to opium alkaloids. It was officially decontrolled in January 2015. It was reclassified as a prescription drug after the FDA and DEA concluded that the impermeability of the blood–brain barrier to this compound made it non-habit-forming, and so without the potential for abuse.[10] Medical useNaloxegol is indicated for the treatment of opioid-induced constipation (OIC) in people with chronic non-cancer pain.[9][11] Side effectsThe most common side effects are abdominal pain, diarrhea, nausea, flatulence, vomiting, and headache.[9] Pharmacodynamic propertiesNaloxegol inhibits opioid binding in μ-opioid receptors in the gastrointestinal tract, thus decreasing the constipating effects (slowing of gastrointestinal motility and transit, hypertonicity, increased fluid reabsorption) associated with opioids.[12] If naloxegol is coadministered with other opioid antagonists, there is a potential for additive effect and increased risk of opioid withdrawal.[9] Mechanism of actionChemically, naloxegol is a pegylated (polyethylene glycol-modified) derivative of α-naloxol. Specifically, the 6-α-hydroxyl group of α-naloxol is connected via an ether linkage to the free hydroxyl group of a monomethoxy-terminated n=7 oligomer of PEG, shown extending at the lower left of the molecule image at right. The "n=7" defines the number of two-carbon ethylenes, and so the chain length, of the attached PEG chain, and the "monomethoxy" indicates that the terminal hydroxyl group of the PEG is "capped" with a methyl group.[13] The pegylation of the 6-α-hydroxyl side chain of naloxol prevents the drug from crossing the blood–brain barrier (BBB).[8] References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||