|
Lithium tantalate
Wikinews has related news:
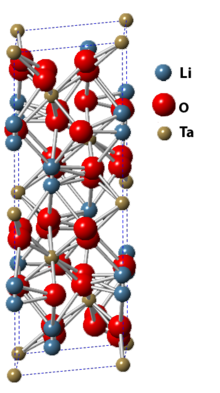
Lithium tantalate is the inorganic compound with the formula LiTaO3. It is a white, diamagnetic, water-insoluble solid. The compound has the perovskite structure. It has optical, piezoelectric, and pyroelectric properties. Considerable information is available from commercial sources about this material.[2] Synthesis and processingLithium tantalate is produced by treating tantalum(V) oxide with lithium oxide. The use of excess alkali gives water-soluble polyoxotantalates. Single crystals of Lithium tantalate are pulled from the melt using the Czochralski method.[2] ApplicationsLithium tantalate is used for nonlinear optics, passive infrared sensors such as motion detectors, terahertz generation and detection, surface acoustic wave applications, cell phones. Lithium tantalate is a standard detector element in infrared spectrophotometers.[3] ResearchThe phenomenon of pyroelectric fusion has been demonstrated using a lithium tantalate crystal producing a large enough charge to generate and accelerate a beam of deuterium nuclei into a deuterated target resulting in the production of a small flux of helium-3 and neutrons through nuclear fusion without extreme heat or pressure.[4] A difference between positively and negatively charged parts of pyroelectric LiTaO3 crystals was observed when water freezes to them.[5] See alsoReferences
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||