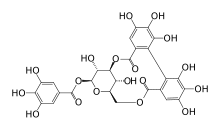
Corilagin
Chemical structure of corilagin
Names
IUPAC name
[3,5-dihydroxy-2-(3,4,5-trihydroxybenzoyl)oxy-6-[(3,4,5-trihydroxybenzoyl)oxymethyl]oxan-4-yl] 3,4,5-trihydroxybenzoate
Other names
Identifiers
ChEMBL
ChemSpider
UNII
InChI=1S/C27H22O18/c28-9-1-6(2-10(29)16(9)32)24(39)45-27-22(38)23-19(35)13(43-27)5-42-25(40)7-3-11(30)17(33)20(36)14(7)15-8(26(41)44-23)4-12(31)18(34)21(15)37/h1-4,13,19,22-23,27-38H,5H2/t13-,19-,22-,23+,27+/m1/s1
N Key: TUSDEZXZIZRFGC-XIGLUPEJSA-N
N InChI=1/C27H24O18/c28-11-1-8(2-12(29)18(11)34)24(39)42-7-17-21(37)23(44-25(40)9-3-13(30)19(35)14(31)4-9)22(38)27(43-17)45-26(41)10-5-15(32)20(36)16(33)6-10/h1-6,17,21-23,27-38H,7H2/t17-,21-,22-,23+,27+/m1/s1
Key: RNKMOGIPOMVCHO-SJMVAQJGBQ
O[C@@H]1[C@H]2COC(=O)c3cc(O)c(O)c(O)c3c4c(O)c(O)c(O)cc4C(=O)O[C@@H]1[C@@H](O)[C@H](OC(=O)c5cc(O)c(O)c(O)c5)O2
c1c(cc(c(c1O)O)O)C(=O)OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H](O2)OC(=O)c3cc(c(c(c3)O)O)O)O)OC(=O)c4cc(c(c(c4)O)O)O)O
Properties
C27 H22 O18
Molar mass
634.45 g/mol
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Corilagin is an ellagitannin . Corilagin was first isolated in 1951 from Dividivi extract and from Caesalpinia coriaria [ 1] [ 2] Alchornea glandulosa Punica granatum [ 3]
It is a weak carbonic anhydrase inhibitor .[ 4]
Ellagic acid and corilagin inhibit TGF-β1 –dependent EMT and has been shown to attenuate fibrogenesis in a mouse model.[ 5] Fibrosis is also indicated in many health conditions, including skin aging and MRSA susceptibility.[ 6]
References
^ Schmidt OT, Lademann R (1951). "Corilagin, ein weiterer kristallisierter Gerbstoff aus Dividivi. X. Mitteilung über natürliche Gerbstoffe". Justus Liebigs Annalen der Chemie . 571 (3): 232– 237. doi :10.1002/jlac.19515710305 . ^ Schmidt OT, Schmidt DM (1952). "Die Umwandlung von Chebulagsäure in Corilagin XIV. Mitteilung über natürliche Gerbstoffe". Justus Liebigs Annalen der Chemie . 578 : 25– 30. doi :10.1002/jlac.19525780105 . ^ Tanaka T, Nonaka GI, Nishioka I (1985). "Punicafolin, an ellagitannin from the leaves of Punica granatum". Phytochemistry . 24 (9): 2075– 2078. Bibcode :1985PChem..24.2075T . doi :10.1016/S0031-9422(00)83125-8 . ^ Satomi H, Umemura K, Ueno A, Hatano T, Okuda T, Noro T (August 1993). "Carbonic anhydrase inhibitors from the pericarps of Punica granatum L" . Biological & Pharmaceutical Bulletin . 16 (8): 787– 90. doi :10.1248/bpb.16.787 PMID 8220326 . ^ Wei Y, Kim TJ, Peng DH, Duan D, Gibbons DL, Yamauchi M, et al. (October 2017). "Fibroblast-specific inhibition of TGF-β1 signaling attenuates lung and tumor fibrosis" . The Journal of Clinical Investigation . 127 (10): 3675– 3688. doi :10.1172/JCI94624 . PMC 5617667 PMID 28872461 . ^ "Researchers identify how skin ages, loses fat and immunity" . medicalxpress.com . Retrieved 5 January 2019 .
Moieties Lactones Monomers
Acetonyl geraniin Alnusiin Bicornin Carlesiin Casuarictin Emblicanin A and B Euscaphinin Galloyl pedunculagin Grandinin Helioscopinin B Jolkinin Lagerstannin A , B and C Macranganin Myrobalanitannin Nupharin A , B , C , D , E and F Pedunculagin Punicalagin Punigluconin Phyllanemblinin A , B , C , D , E and F Punicalin Roburin E Rugosin E Sanguiin H-5 Stenophyllanin A , B and C Strictinin Tellimagrandin I and II Teracatain Terchebulin Terflavin A and B Tergallic acid Tergallic acid dilactone
Oligomers Other