|
Bulevirtide
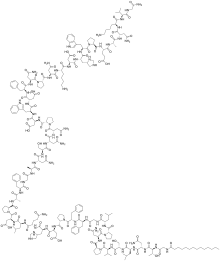
Bulevirtide, sold under the brand name Hepcludex, is an antiviral medication for the treatment of chronic hepatitis D (in the presence of hepatitis B).[6] The most common side effects include raised levels of bile salts in the blood and reactions at the site of injection.[6] Bulevirtide works by attaching to and blocking a receptor (target) through which the hepatitis delta and hepatitis B viruses enter liver cells.[6] By blocking the entry of the virus into the cells, it limits the ability of HDV to replicate and its effects in the body, reducing symptoms of the disease.[6] Bulevirtide was approved for medical use in the European Union in July 2020.[6] Structural formulaBulevirtide is a 47-amino acid peptide with the following sequence:[8] CH3(CH2)12CO-Gly-Thr-Asn-Leu-Ser-Val-Pro-Asn-Pro-Leu-Gly-Phe-Phe-Pro-Asp-His-Gln-Leu-Asp-Pro-Ala-Phe-Gly-Ala-Asn-Ser-Asn-Asn-Pro-Asp-Trp-Asp-Phe-Asn-Pro-Asn-Lys-Asp-His-Trp-Pro-Glu-Ala-Asn-Lys-Val-Gly-NH2 (C13H27CO-GTNLSVPNPLGFFPDHQLDPAFGANSNNPDWDFNPNKDHWPEANKVG-NH2) Medical usesBulevirtide is indicated for the treatment of chronic hepatitis delta virus (HDV) infection in plasma (or serum) HDV-RNA positive adult patients with compensated liver disease.[6][9] PharmacologyMechanism of actionBulevirtide binds and inactivates the sodium/bile acid cotransporter, blocking both HBV and HDV viruses from entering hepatocytes.[10] The hepatitis B virus uses its surface lipopeptide pre-S1 for docking to mature liver cells via their sodium/bile acid cotransporter (NTCP) and subsequently entering the cells. Myrcludex B is a synthetic N-acylated pre-S1[11][12] that can also dock to NTCP, blocking the virus's entry mechanism.[13] The drug is also effective against hepatitis D because the hepatitis D virus uses the same entry receptor as HBV and is only effective in the presence of a hepatitis B virus infection.[13] Pre-clinical data in mice suggests that pharmacological inhibition of NTCP-mediated bile salt uptake may also be effective to lower hepatic bile salt accumulation in cholestatic conditions. This reduces hepatocellular damage.[14] An increased ratio of phospholipid to bile salts seen in bile upon NTCP inhibition may further contribute to the protective effect as bile salts are less toxic in presence of phospholipids.[15] References
|
||||||||||||||||||||||||||||||||||||||||||