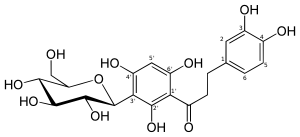
Aspalathin
Names
IUPAC name
3-(3,4-Dihydroxyphenyl)-1-[5-(β-D -glucopyranosyl)-2,4-dihydroxyphenyl]propan-1-one
Systematic IUPAC name
3-(3,4-Dihydroxyphenyl)-1-{2,4-dihydroxy-5-[(2S ,3R ,4R ,5S ,6R )-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]phenyl}propan-1-one
Identifiers
ChEBI
ChemSpider
ECHA InfoCard 100.233.299
InChI=1S/C21H24O11/c22-7-14-17(28)19(30)20(31)21(32-14)16-13(27)6-12(26)15(18(16)29)10(24)4-2-8-1-3-9(23)11(25)5-8/h1,3,5-6,14,17,19-23,25-31H,2,4,7H2/t14-,17-,19+,20-,21+/m1/s1
N Key: VCPUQYKWJRESOC-VJXVFPJBSA-N
N InChI=1/C21H24O11/c22-7-14-17(28)19(30)20(31)21(32-14)16-13(27)6-12(26)15(18(16)29)10(24)4-2-8-1-3-9(23)11(25)5-8/h1,3,5-6,14,17,19-23,25-31H,2,4,7H2/t14-,17-,19+,20-,21+/m1/s1
Key: VCPUQYKWJRESOC-VJXVFPJBBW
O=C(c1c(O)c(c(O)cc1O)[C@@H]2O[C@@H]([C@@H](O)[C@H](O)[C@H]2O)CO)CCc3ccc(O)c(O)c3
Properties
C 21 H 24 O 11
Molar mass
−1
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Aspalathin is a C -linked dihydrochalcone glucoside found in rooibos tea , a herbal tea prepared from the South African rooibos plant , Aspalathus linearis Fabaceae ).[ 1]
It was first isolated in 1965 by chromatography .[ 2]
It has demonstrated antidiabetic activity.[ 3]
References
^ Bramati L; et al. (2002). "Quantitative Characterization of Flavonoid Compounds in Rooibos Tea (Aspalathus linearis ) by LC-UV/DAD". Journal of Agricultural and Food Chemistry . 50 (20). Elsevier: 5513– 5519. doi :10.1021/jf025697h . PMID 12236672 . ^ Koeppen, B. H.; Roux, D. G. (June 1966). "C-Glycosylflavonoids. The chemistry of aspalathin" . Biochemical Journal . 99 (3): 604– 609. doi :10.1042/bj0990604 . ISSN 0264-6021 . PMC 1265048 PMID 4290475 . ^ Bader, Michael; Mazibuko-Mbeje, Sithandiwe E.; Dludla, Phiwayinkosi V.; Johnson, Rabia; Joubert, Elizabeth; Louw, Johan; Ziqubu, Khanyisani; Tiano, Luca; Silvestri, Sonia; Orlando, Patrick; Opoku, Andy R.; Muller, Christo J. F. (2019). "Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration" . PLOS ONE . 14 (5): e0216172. Bibcode :2019PLoSO..1416172M . doi :10.1371/journal.pone.0216172 ISSN 1932-6203 . PMC 6497260 PMID 31048842 .
External links