|
AlkanolamineIn organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl (−OH) and amino (−NH2, −NHR, and −NR2) functional groups on an alkane backbone. Most alkanolamines are colorless.[1][citation needed]
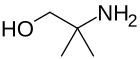
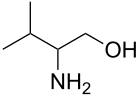
1-Aminoalcohols1-Aminoalcohols are better known as hemiaminals. Methanolamine is the simplest member. 2-Aminoalcohols2-Aminoalcohols are an important class of organic compounds that are often generated by the reaction of amines with epoxides:
Simple alkanolamines are used as solvents, synthetic intermediates, and high-boiling bases.[2] Hydrogenation or hydride reduction of amino acids gives the corresponding 2-aminoalcohols. Examples include prolinol (from proline), valinol (from valine), tyrosinol (from tyrosine). Key members: ethanolamine, dimethylethanolamine, N-methylethanolamine, Aminomethyl propanol. Two popular drugs, often called alkanolamine beta blockers, are members of this structural class: propranolol, pindolol. Isoetarine is yet another medicinally useful derivative of ethanolamine.[citation needed] 1,3-, 1,4-, and 1,5-amino alcohols
Natural productsMost proteins and peptides contain both alcohols and amino groups. Two amino acids are alkanolamines, formally speaking: serine and hydroxyproline.
References
External links
|