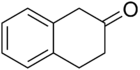
2-Tetralone
Names
Preferred IUPAC name
3,4-Dihydronaphthalen-2(1H )-one
Other names
β-Tetralone; 2-Tetralone
Identifiers
ChemSpider
ECHA InfoCard 100.007.727
EC Number
UNII
InChI=1S/C10H10O/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-4H,5-7H2
N Key: KCKZIWSINLBROE-UHFFFAOYSA-N
N InChI=1/C10H10O/c11-10-6-5-8-3-1-2-4-9(8)7-10/h1-4H,5-7H2
Key: KCKZIWSINLBROE-UHFFFAOYAE
Properties
C 10 H 10 O
Molar mass
−1
Appearance
Colourless liquid
Density
1.106 g/mL
Melting point
18 °C (64 °F; 291 K)
Boiling point
70–71 °C (158–160 °F; 343–344 K) /0.25 mm
in basic water
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
2-Tetralone is an organic chemical compound with the molecular formula C10 H10 O. This colourless oil is an intermediate in organic synthesis . It is a ketone derivative of tetralin , a hydrogenated derivative of naphthalene .
History and synthesis
The compound was first obtained by Eugen Bamberger and Wilhelm Lodter in 1893 by dehydrohalogenation of 3-chloro-2-tetralol with hot alkali.[ 1] [ 2]
2-Tetralone may be prepared by reductive cleavage of 2-naphthyl ethers.[ 3]
Applications
2-Tetralone is an intermediate in the synthesis of a variety of pharmaceutical drugs including L-687,384 , nepinalone , napamezole ,[ 4] spirodone , and trioxifene .
See also
References
^ Shner, V. F.; Przhiyaglovskaya, N. M. (1966-07-31). "COMPOUNDS OF THE β-TETRALONE SERIES" . Russian Chemical Reviews . 35 (7): 523. doi :10.1070/RC1966v035n07ABEH001493 . ISSN 0036-021X . ^ Bamberger, Eug.; Lodter, W. (1893). "Ueber das Dihydronaphtalin und einige seiner Derivate" . Berichte der deutschen chemischen Gesellschaft (in German). 26 (2): 1833– 1844. doi :10.1002/cber.189302602133 . ISSN 1099-0682 . ^ M. D. Soffer, M. P. Bellis, Hilda E. Gellerson, and Roberta A. Stewart "β-Tetralone" Org. Synth. 1952, 32, 97 doi :10.15227/orgsyn.032.0097
^ Wentland, Mark P.; Bailey, Denis M.; Alexander, E. John; Castaldi, Michael J.; Ferrari, Richard A.; Haubrich, Dean R.; Luttinger, Daniel A.; Perrone, Mark H. (1987). "Synthesis and antidepressant properties of novel 2-substituted 4,5-dihydro-1H-imidazole derivatives". Journal of Medicinal Chemistry . 30 (8): 1482– 1489. doi :10.1021/jm00391a034 . PMID 3039138 .